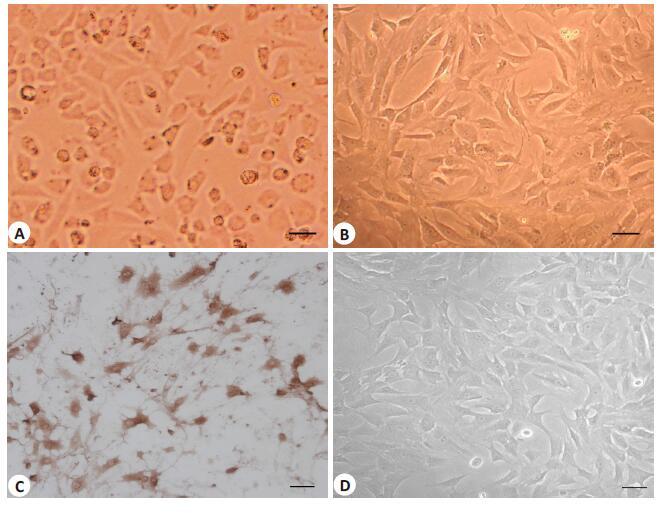
Inflammatory responses of human osteoarthritic chondrocytes induced by interleukin 18
-
摘要:
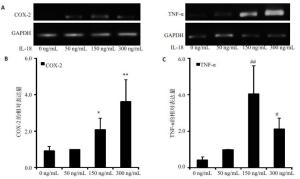
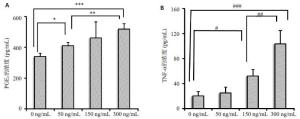
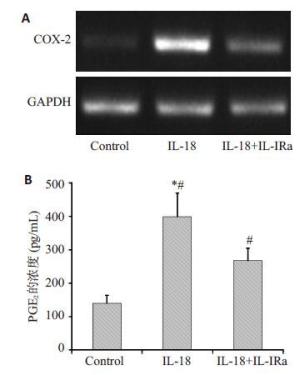
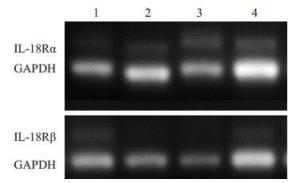
目的探讨IL-18 对骨关节炎软骨细胞的作用。 方法取骨关节炎患者的膝关节软骨,进行原代细胞培养。不同浓度的IL-18(0,50,150,300 ng/mL)刺激软骨细胞,RT-PCR检测TNFα和COX-2 mRNA的表达,ELISA检测TNFα、PGE2的水平。应用IL-1 受体阻断剂(IL-1Ra)+IL-18 干预软骨细胞,检测软骨细胞COX-2 的表达量和培养液中PGE2的水平。提取软骨细胞RNA,测定IL-18受体的表达。 结果对于COX-2和TNFα,300 ng/mL组和150 ng/mL组mRNA的表达量显著高于对照组(P<0.01,P<0.05)。50、300 ng/mL组的PGE2水平显著高于对照组(P<0.05)。150、300 ng/mL组的TNFα蛋白的浓度显著高于对照组(P<0.05),IL-1Ra 组的PGE2浓度高于对照组(P<0.01),但是低于IL-18 组(P<0.01)。软骨细胞能够检测到IL-18 受体的表达。 结论IL-18诱导软骨细胞产生炎症应答,这种作用和IL-1β有关但不完全依赖IL-1β。 Abstract:Objective To explore the role of interleukin 18(IL-18) on the osteoarthritis (OA) patients' chondrocytes. Methods Knee cartilage samples were obtained from osteoarthritic patients, and the primary cells were cultured. After stimulated by IL-18 (0, 50, 150, 300 ng/mL), chondrocytes were collect to obtain the total RNA, and the supernatant were extracted, too. The mRNA’s expression of COX-2 and TNFα were detected by quantitative RT-PCR and the level of PGE2 and TNFα were investigated using ELISA. Besides IL-18, IL-1 receptor inhibitor (IL-1Ra) was also added into the medium of cell culture. COX-2 mRNA and PGE2 were respectively determination. IL-18 receptors in chondrocytes were detected by RT-PCR. Results mRNA expression of COX-2 and TNFα in 150 and 300 ng/mLwere both significantly higher than that in control group (P<0.05). The level of in 50 and 300 ng/mL group were higher than that in control group (P<0.01). While the concentration of TNFα in 150 and 300 ng/mL groups appeared more than that in control group significantly (P<0.01). And the density of PGE2 in IL-Ra group was significantly more than that in control group, but less than that of IL-18 group. IL-18R can be detected on chondrocytes. Conclusions IL-18 induces inflammatory responses in osteoarthritic chondrocytes and that this effect was related with, although not entirely dependent on, IL-1β. -
Key words:
- osteoarthritis /
- interleukin 18 /
- chondrocytes /
- degradation
-
表 1 IL-18 干预后PGE2、TNF-α的水平(x±s, pg/mL)
Group PGE2 (n=8) TNF-α (n=8) 0 ng/mL 340.47±22.03 20.48±7.36 50 ng/mL 410.03±22.97* 25.32±9.55 150 ng/mL 460.38±112.86* 52.45±10.93# 300 ng/mL 518.28±38.80* 103.80±22.29# *P<0.01 vs PGE2 in 0 ng/mL group; #P<0.01 vs TNF-α in 0 ng/mL group. -
[1] "Nho SJ, Kymes SM, Callaghan JJ, et al. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations[J]. J Am Acad Orthop Surg, 2013, 21(Suppl 1): S1-6. [2] Bitton R. The economic burden of osteoarthritis[J]. Am J Manag Care, 2009, 15(8 Suppl): S230-5. [3] 那键, 刘艺, 马克勇, 等. 老年性骨关节炎的分子生物学机制及治疗 展望[J]. 中国老年学杂志, 2010, 30(20): 3035-7. [4] Shao XT, Feng L, Gu LJ, et al. Expression of interleukin-18, IL-18BP, and IL-18R in serum, synovial fluid, and synovial tissue in patients with rheumatoid arthritis[J]. Clin Exp Med, 2009, 9(3): 215-21. [5] Moser C. Response to: cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection[J]. Arthritis Res Ther, 2010, 12(6): 410-1. [6] Smith MD, Triantafillou S, Parker A, et al. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis[J]. J Rheumatol, 1997, 24(2): 365-71. [7] Dinarello CA. The IL-1 family and inflammatory diseases[J]. Clin Exp Rheumatol, 2004, 20(5 Suppl 27): S1-13. [8] Dai SM, Shan ZZ, Xu H, et al. Cellular targets of interleukin-18 in rheumatoid arthritis[J]. Ann Rheum Dis, 2007, 66(11): 1411-8. [9] Mohtai M, Gupta MK, Donlon B, et al. Expression of interleukin-6 in osteoarthritic chondrocytes and effects of fluid-induced shear on this expression in normal human chondrocytes in vitro[J]. J Orthop Res, 1996, 14(1): 67-73. [10] Bjornsson GL, Thorsteinsson L, Gudmundsson KO, et al. Inflammatory cytokines in relation to adrenal response following total hip replacement [J]. Scand J Immunol, 2007, 65(1): 99-105. [11] Ghayur T, Banerjee S, Hugunin M, et al. Caspase-1 processes IFNgamma- inducing factor and regulates LPS-induced IFN-gamma production[J]. Nature, 1997, 386(6625): 619-23. [12] Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells[J]. Nature, 1995, 378(6552): 88-91. [13] Pawlik A, Kurzawski M, Drozdzik M, et al. Interleukin-18 gene (IL18) promoter polymorphisms in patients with rheumatoid arthritis[J]. Scand J Rheumatol, 2009, 38(3): 159-65. [14] Matsui K, Tsutsui H, Nakanishi K. Pathophysiological roles for IL-18 in inflammatory arthritis[J]. Expert Opin Ther Targets, 2003, 7(6): 701-24. [15] Olee T, Hashimoto S, Quach J, et al. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses [J]. J Immunol, 1999, 162(2): 1096-100. [16] Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method[J]. Methods, 2001, 25(4): 402-8. [17] Möller B. Interleukin-18 receptor expression in synovial fluidderived fibroblast-like synoviocytes: comment on the article by Kawashima and Miossec[J]. Arthritis Rheum, 2004, 50(7): 2373-4. [18] Li X, Ellman M, Muddasani P, et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis [J]. Arthritis Rheum, 2009, 60(2): 513-23. [19] Maganev VA, Davletshin RA, Davletshina GK, et al. Dynamics of tumour necrosis factor-alpha and clinical signs of osteoarthrosis during the treatment with alflutop in combination with peloid applications under conditions of health resort[J].Vopr kurortol fizioter lech Fiz Kult, 2011(2): 18-20. [20] Orita S, Koshi T, Mitsuka T, et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee[J]. BMC Musculoskelet Disord, 2011, 12 (1): 144-7. [21] Chan BY, Fuller ES, Russell AK, et al. Increased chondrocyte sclerostin May protect against cartilage degradation in osteoarthritis [J]. Osteoar Cartilage, 2011, 19(7): 874-85. [22] Kawashima M, Miossec P. Heterogeneity of response of rheumatoid synovium cell subsets to interleukin-18 in relation to differential interleukin-18 receptor expression[J]. Arthritis Rheum, 2003, 48(3): 631-7. [23] Notoya K, Jovanovic DV, Reboul P, et al. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E-2 via the induction of cyclooxygenase-2[J]. J Immunol, 2000, 165(6): 3402-10. [24] Singh R, Ahmed S, Malemud CJ, et al. Epigallocatechin-3-gallate selectively inhibits interleukin-1beta-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes[J]. J Orthop Res, 2003, 21(1): 102-9. [25] Lim H, Kim HP. Matrix metalloproteinase-13 expression in IL-1β-treated chondrocytes by activation of the p38 MAPK/c-Fos/ AP-1 and JAK/STAT pathways[J]. Arch Pharm Res, 2011, 34(1): 109-17. [26] Lee JK, Kim SH, Lewis EC, et al. Differences in signaling pathways by IL-1beta and IL-18[J]. Proc Natl Acad Sci USA, 2004, 101(23): 8815-20. [27] Chandrasekar B, Mummidi S, Mahimainathan L, et al. Interleukin- 18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase- 9 expression and is inhibited by atorvastatin[J]. J Biol Chem, 2006, 281(22): 15099-109. [28] 王凤龙, 江建明, 肖军, 等. 白细胞介素-18在骨关节炎滑膜细胞中的 表达及意义[J]. 中华风湿病学杂志, 2010, 14(4): 260-2. [29] Inoue H, Hiraoka K, Hoshino T, et al. High levels of serum IL-18 promote cartilage loss through suppression of aggrecan synthesis [J]. Bone, 2008, 42(6): 1102-10." -






 下载:
下载: