Clinical value of amide proton transfer weighted imaging in predicting Ki67 proliferation level of glioma
-
摘要:
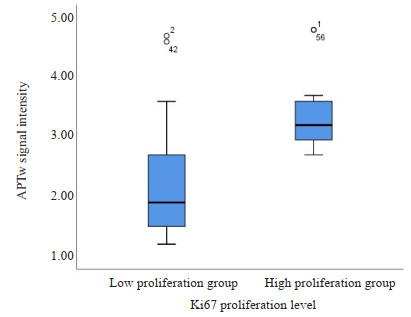
目的 探讨酰胺质子转移成像(APTw)与脑胶质瘤Ki67细胞增殖指数的相关性,并分析其预测的准确度。 方法 收集来自北京天坛医院的63例经手术病理证实且于术前2周内进行APTw扫描的脑胶质瘤患者,最终共纳入59例脑胶质瘤患者。按照免疫组化中获取的Ki67细胞增殖指数,将患者分为低增殖组(细胞增殖指数 < 20%,n=39)和高增殖组(细胞增殖指数≥20%,n=20),并测量两组肿瘤APTw信号强度。采用Pearson检验分析APTw与Ki67细胞增殖指数之间的相关性;采用Mann-Whitney U检验分析APTw信号强度在两增殖组间的差异;以APTw信号强度为影像标志物建立预测模型来预测脑胶质瘤Ki67细胞增殖水平,绘制ROC曲线评估其诊断效能,最后计算约登指数获取APTw信号强度的临界值。 结果 APTw信号强度与Ki67细胞增殖指数之间呈正相关(r=0.629,P < 0.001);APTw信号强度在两个Ki67增殖水平组间差异有统计学意义(Z=4.539,P < 0.001);该预测模型的曲线下面积为0.863(95% CI:0.770~0.956),敏感度为1,特异性为0.718;约登指数结果显示该预测模型中APTw信号强度的临界值为2.55。 结论 APTw信号强度可以作为影像标志物来预测脑胶质瘤Ki67细胞增殖水平,并且具有良好的诊断效能及敏感性;当APTw信号强度大于2.55时,脑胶质瘤Ki67细胞增殖水平更倾向于高增殖水平,反之,更倾向于低增殖水平。 -
关键词:
- 脑胶质瘤 /
- Ki67细胞增殖水平 /
- 酰胺质子转移成像 /
- 影像标志物
Abstract:Objective To explore the correlation between amide proton transfer imaging (APTw) and the Ki67 proliferation index of glioma and analyze the accuracy of the predicting model. Methods 63 glioma patients from Beijing Tiantan Hospital were accepted APTw imaging within 2 weeks before surgery, and 59 glioma patients were finally included. The patients were divided into two groups according to immunohistochemistry results: low proliferation group (cell proliferation index < 20%, n=39) and high proliferation group (cell proliferation index 20%, n=20), and the mean APTw signal intensities of gliomas were measured. The correlaction between APTw signal intensity and Ki67 proliferation index was evaluated by Pearson test. The differences of mean APTw signal intensity between two proliferation groups were evaluated by Mann-Whitney U test. The predicting model by mean APTw signal intensity as an imaging biomarker was established, and then ROC curve was drawn to assess their diagnostic performance. Finally, the cut-off value of the APTw signal intensity for this model was obtained by Yuden index. Result There was a positivity correlaction between APTw signal intensity and Ki67 proliferation index (r=0.629, P < 0.001), and there was a significant difference between two proliferation groups (Z=4.539, P < 0.001). The AUC for predicting model was 0.863 (95% CI: 0.770-0.956), with sensitivity of 1, and specificity of 0.718. Youden index showed the cut-off value was 2.55. Conclusion APTw signal intensity can be served as an imaging biomarker for the predicting model of glioma Ki-67 proliferation level, which has a good diagnostic effectiveness and good sensitivity; When the mean APTw signal intensity is greater than 2.55, the glioma prefers high proliferation level, otherwise, the glioma prefers low proliferation level. -
Key words:
- glioma /
- Ki-67 proliferation level /
- amide proton transfer imaging /
- biomarker
-
图 2 脑胶质瘤常规MRI、APTw及免疫组化染色图
Figure 2. Conventional MRI, APTw and immunohistochemical result of brain glioma. Oligodendroglioma, WHO Ⅱ, located in the frontotemporal lobe. T2w showed high signal, uniform distribution, clear margin, no cyst, necrosis, edema, no ventricle compression, superficial groove fission, local scalp tissue swelling (A), and no obvious enhancement (B); C: APTw showed a low signal (1.54%); D: Immunohistochemical result (DAB staining, 100 micron) showed Ki67 low proliferation level (cell staining rate < 20%).
图 3 脑胶质瘤MRI图及免疫组化染色图
Figure 3. Conventional MRI, APTw and immunohistochemical result of brain glioma. Glioblastoma, WHO Ⅳ, located in the frontotemporal lobe and thalamus. T2w showed high signal, less uniform distribution, blurred margin, cystic changes, necrosis, peritumoral edema, midline right deviation, left ventricle compression, shallow sulcus fission, local scalp tissue swelling (A), and enhancement (B); C: APTw showed a high signal (4.29%); D: Immunohistochemical result (DAB staining, 100 micron) showed high Ki67 proliferation level (cell staining rate> 20%).
表 1 不同细胞增殖水平患者的一般资料及组间的比较结果
Table 1. General data of the patients with different proliferation levels and comparative results between the two groups.
Index Low proliferation level (n=39) High proliferation level (n=20) Z/χ2 P Age(years, Mean±SD) 44.02±13.22 49.75±8.69 -1.827 0.068 Gender (n) Male 18 10 0.078 0.779 Female 21 10 APTw signal intensity(%,Mean±SD) 2.21±0.89 3.37±0.59 4.539 <0.001 -
[1] Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011[J]. Neuro-oncology, 2014, 16(Suppl 4): iv1-iv63. doi: 10.1093/neuonc/nou223 [2] Wesseling P, Capper D. WHO 2016 classification of gliomas[J]. Neuropathol Appl Neurobiol, 2018, 44(2): 139-50. doi: 10.1111/nan.12432 [3] Gerdes J, Lemke H, Baisch H, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67[J]. J Immunol, 1984, 133(4): 1710-5. doi: 10.4049/jimmunol.133.4.1710 [4] Yoshida Y, Nakada M, Harada T, et al. The expression level of sphingosine-1-phosphate receptor type 1 is related to MIB-1 labeling index and predicts survival of glioblastoma patients[J]. J Neurooncol, 2010, 98(1): 41-7. doi: 10.1007/s11060-009-0064-5 [5] Hu XH, Miao W, Zou YJ, et al. Expression of p53, epidermal growth factor receptor, Ki-67 and O6-methylguanine-DNA methyl-transferase in human gliomas[J]. Oncol Lett, 2013, 6(1): 130-4. doi: 10.3892/ol.2013.1317 [6] Deacu M, Popescu S, Docu Axelerad A, et al. Prognostic factors of low-grade gliomas in adults[J]. Curr Oncol, 2022, 29(10): 7327-42 doi: 10.3390/curroncol29100576 [7] Sun XM, Kaufman PD. Ki-67: more than a proliferation marker[J]. Chromosoma, 2018, 127(2): 175-86. doi: 10.1007/s00412-018-0659-8 [8] Arshad H, Ahmad Z, Hasan SH. Gliomas: correlation of histologic grade, Ki67 and p53 expression with patient survival[J]. Asian Pac J Cancer Prev, 2010, 11(6): 1637-40. [9] Yang ZX, Ling F, Ruan SB, et al. Clinical and prognostic implications of 1p/19q, IDH, BRAF, MGMT promoter, and TERT promoter alterations, and expression of ki-67 and p53 in human gliomas[J]. Cancer Manag Res, 2021, 13: 8755-65. doi: 10.2147/CMAR.S336213 [10] 朱雪超, 何玉麟, 邬莺莺, 等. 基于T2WI的影像组学模型预测胶质母细胞瘤Ki-67的表达水平[J]. 磁共振成像, 2021, 12(9): 53-6. https://www.cnki.com.cn/Article/CJFDTOTAL-CGZC202109012.htm [11] Sun XJ, Pang PP, Lou L, et al. Radiomic prediction models for the level of Ki-67 and p53 in glioma[J]. J Int Med Res, 2020, 48(5): 300060520914466. [12] Zhou JY, Lal B, Wilson DA, et al. Amide proton transfer (APT) contrast for imaging of brain tumors[J]. Magn Reson Med, 2003, 50(6): 1120-6. doi: 10.1002/mrm.10651 [13] Zhou JY. Amide proton transfer imaging of the human brain[J]. Methods Mol Biol, 2011, 711: 227-37. [14] Guo H, Liu J, Hu JJ, et al. Diagnostic performance of gliomas grading and IDH status decoding A comparison between 3D amide proton transfer APT and four diffusion-weighted MRI models[J]. J Magn Reson Imaging, 2022, 56(6): 1834-44. doi: 10.1002/jmri.28211 [15] Jiang SS, Rui QH, Wang Y, et al. Discriminating MGMT promoter methylation status in patients with glioblastoma employing amide proton transfer-weighted MRI metrics[J]. Eur Radiol, 2018, 28(5): 2115-23. doi: 10.1007/s00330-017-5182-4 [16] Zhuo ZZ, Qu LY, Zhang P, et al. Prediction of H3K27M-mutant brainstem glioma by amide proton transfer-weighted imaging and its derived radiomics[J]. Eur J Nucl Med Mol Imaging, 2021, 48 (13): 4426-36. doi: 10.1007/s00259-021-05455-4 [17] Zhang N, Zhang HN, Gao BB, et al. 3D amide proton transfer weighted brain tumor imaging with compressed SENSE: effects of different acceleration factors[J]. Frontiers in Neuroscience, 2022, 16: 876587. doi: 10.3389/fnins.2022.876587 [18] 李欣蓓, 宋玉坤, 朱筱磊, 等. 氨基质子转移MRI对脑胶质瘤分级及预测肿瘤细胞增殖的诊断价值[J]. 放射学实践, 2017, 32(4): 355-9. https://www.cnki.com.cn/Article/CJFDTOTAL-FSXS201704016.htm [19] 谢新凤. 酰胺质子转移成像预测胶质瘤分级和Ki-67表达状态的研究[D]. 广州: 南方医科大学, 2019. [20] 谢聪, 段云云, 王晓波, 等. MR酰胺质子转移成像预测脑干胶质瘤病理分级的价值[J]. 中华放射学杂志, 2022, 56(2): 163-7. [21] Reavey-Cantwell JF, Haroun RI, Zahurak M, et al. The prognostic value of tumor markers in patients with glioblastoma multiforme: analysis of 32 patients and review of the literature[J]. J Neurooncol, 2001, 55(3): 195-204. doi: 10.1023/A:1013845004294 [22] Togao O, Yoshiura T, Keupp J, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades[J]. Neuro Oncol, 2014, 16(3): 441-8. doi: 10.1093/neuonc/not158 [23] Su C, Liu C, Zhao L, et al. Amide proton transfer imaging allows detection of glioma grades and tumor proliferation: comparison with ki-67 expression and proton MR spectroscopy imaging[J]. AJNR Am J Neuroradiol, 2017, 38(9): 1702-9. doi: 10.3174/ajnr.A5301 [24] Jiang SS, Eberhart CG, Zhang Y, et al. Amide proton transfer-weighted magnetic resonance image-guided stereotactic biopsy in patients with newly diagnosed gliomas[J]. Eur J Cancer, 2017, 83: 9-18. doi: 10.1016/j.ejca.2017.06.009 [25] Bai Y, Lin YS, Zhang W, et al. Noninvasive amide proton transfer magnetic resonance imaging in evaluating the grading and cellularity of gliomas[J]. Oncotarget, 2017, 8(4): 5834-42. doi: 10.18632/oncotarget.13970 [26] Su CL, Jiang JJ, Zhang S, et al. Radiomics based on multicontrast MRI can precisely differentiate among glioma subtypes and predict tumour-proliferative behaviour[J]. Eur Radiol, 2019, 29(4): 1986-96. doi: 10.1007/s00330-018-5704-8 -







 下载:
下载: