Clinical characteristics and risk factors of tumor-like calcinosis
-
摘要:
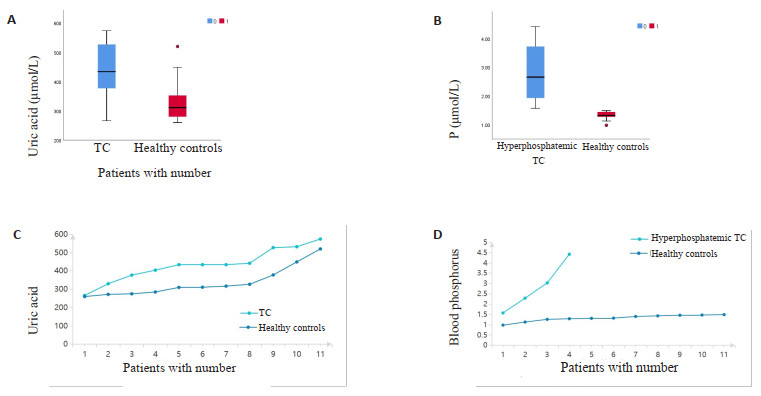
目的 探讨罕见病肿瘤样钙质沉着症(TC)的临床特点并分析疾病相关风险因素。 方法 收集2001年1月~2021年8月就诊于南方医科大学南方医院并经手术病理确诊为TC的患者25例,分析统计临床特点、影像学表现及病理特征。将临床资料齐全的11例住院TC患者与11例年龄、性别匹配的健康对照者进行差异性分析,总结TC患者的生化特征,探讨可能导致该疾病的潜在风险因素。 结果 X线多表现为软组织内的高密度钙化影。MRI表现为短T1长T2信号影,肿块内信号不均,边界清晰,局部可与滑膜囊相连。与健康人群相比,TC患者的血尿酸水平升高(P=0.038),血尿酸水平与TC患病率存在正相关关系,高尿酸导致TC的患病风险增加了3倍(RR=3,95% CI:1.041~8.646);高血磷TC患者的血磷和钙磷乘积较健康人群升高(P < 0.05),高血磷导致TC的患病风险增加了3.2倍(RR=3.2,95% CI:1.547~6.619)。 结论 高血磷和高尿酸增加TC的患病风险,在高尿酸血症和高磷血症患者中需注意鉴别TC,综合临床表现、生化结果、影像学表现、病理诊断使TC得到早期诊治。 Abstract:Objective To investigate the clinical characteristics of patients with tumor-like calcinosis (TC) in rare diseases and analyze the associated risk factors. Methods We collected clinical data and laboratory data on 25 patients with TC at Nanfang Hospital from January 2001 to August 2021. All the patients were diagnosed by surgical pathology. The clinical, imaging and pathological features were analyzed. Eleven hospitalized patients with complete clinical data and eleven age- and gender-matched healthy controls were enrolled into the study. The clinical and biochemical characteristics of TC patients were analyzed and summarized to explore the potential risk factors associated with the development of TC. Results X-ray of patients often showed high-density calcifications in soft tissues and MRI showed short T1 and long T2 signal shadows. The tumor exhibited uneven signal intensity, with clear boundary, and the local areas may be connected to the synovial capsule. Compared with healthy people, the serum uric acid levels were significantly increased in TC patients (P=0.038). The serum uric acid levels were positively correlated with the prevalence of TC, and the risk of TC increased by threefold (RR=3, 95% CI: 1.041-8.646). In addition, the product of serum phosphorus and calcium- phosphorus ratio in TC patients with hyperphosphatemia was increased significantly to the healthy population P < 0.05). Hyperphosphatemia increased the risk of TC by 3.2-fold (RR=3.2, 95% CI: 1.547-6.619). Conclusion High serum phosphate and uric acid increased the risk of TC. Therefore, it was important to make a differential diagnosis between TC patients and patients with hyperuricemia or hyperphosphatemia. A comprehensive consideration, including age of disease onset, blood phosphorus concentration, past medical history, imaging, and pathological diagnosis could help to reduce the rate of misdiagnosis. -
Key words:
- tumoral calcinosis /
- iconography /
- pathologic diagnosis /
- serum phosphorus /
- uric acid
-
图 4 就诊于内分泌科的13岁TC患者治疗前后右侧内眦处及双膝肿块变化情况
Figure 4. The changes of masses in the right inner canthus and knees of 13-year-old TC patients in the Department of Endocrinology before and after treatment were analyzed. A: Right inner canthus lesions before treatment; B: Half a year after treatment, the right eye inner canthus lesions; C: Double knee joint lesions before treatment; D: Bilateral knee joint lesions after treatment.
表 1 TC患者的临床特点分析
Table 1. Analysis of clinical characteristics of TC patients [n(%)]
Clinical features Value P Age(year, Mean) 28 Duration(year, Mean) 4.5 Gender 0.072 Male 17(68.0) Female 8(32.0) Initial symptom < 0.001 Asymptomatic masses / nodules 16(84.2) Joint pain with limited mobility 2(10.5) Skin itching, ulceration, pus 1(5.3) Clinical manifestations at presentation 0.004 Original mass unchanged 11(57.9) Surface skin itching 2(10.5) Redness, swelling, tenderness 2(10.5) Rupture, pus 3(15.8) limitation of movement 1(5.3) Progressive increase of mass 6(31.6) Diseased parts 0.013 Haunch 8(32.0) Scrotum 8(32.0) Knee joint 2(8.0) Hip joint 1(4.0) Knuckle 2(8.0) Inner canthus 3(12.0) Shoulder joint 1(4.0) Number of masses < 0.001 One 20(80) Two 2(8.0) Three and above 3(12.0) Tumor size (Maximum diameter, cm) < 0.001 ≤1 3(12.0) 1-5 16(64.0) ≥5.0 2(8.0) Unknown 4(16.0) Preoperative diagnostic 0.053 Benign tumour 8(32.0) Skin lump 4(16.0) Wen 3(12.0) Myositis ossificans 2(8.0) Dermatitis 1(4.0) Dermatomyositis 2(8.0) Melanotic nevus 1(4.0) TC 1(4.0) Synovial chondromatosis 1(4.0) Unable to diagnose 2(8.0) Pathological staging 0.072 Active stage 17(68.0) Resting stage 8(32.0) 表 2 11例TC住院患者与11例性别、年龄相匹配健康对照的生化结果
Table 2. Biochemical results of 11 TC patients and 11 healthy controls matched in gender and age
Biochemical index TC patients Healthy controls P Serum calcium(mmol/L) 2.31±0.10 2.30±0.08 0.893 Serum phosphorus(mmol/L) 1.46(1.32, 2.66) 1.32(1.26, 1.46) 0.074 HTC 2.66(1.75, 4.07) 1.32(1.26.1.46) 0.001 NHTC 1.36±0.66 1.32±0.16 0.651 Calcium-phosphorus product(mg/dL) 41.64(38.55, 77.95) 38.17(34.87, 42.65) 0.044 HTC 77.95(51.49,106.47) 38.17(34.87,42.65) 0.004 NHTC 38.99±1.55 37.69±4.71 0.563 Creatinine(μmol/L) 77.00(52.50, 314.20) 65.0(52.00, 76.00) 0.370 Uric acid(μmol/L) 434.00(353.50,529.50) 311.00(275.00,378.00) 0.038 eGFR(mL/min) 93.80±37.35 119.15±99.53 0.126 TG (mmol/L) 1.18±0.45 1.82±1.22 0.291 CHOL(mmol/L) 4.04±1.07 4.34±0.57 0.515 HDL-C(mmol/L) 1.11±0.26 1.03±0.16 0.576 LDL-C (mmol/L) 2.74±0.49 2.58±0.85 0.668 HTC: Hyperphosphatemia TC; NHTC: Normal phosphorus TC; eGFR: Glomerular filtration rate; TG: Triglyceride; HDL-C: High density lipoprotein; LDL-C: Low density lipoprotein; 表 3 静止期和活动期的病理特点比较
Table 3. Comparison of pathological characteristics between stationary phase and active phase
Pathological feature Stationary phase Active phase Cell type No cells, only collagen fibers Chronic inflammatory cells multinucleated giant cell, etc. Under the microscope Only collagen fibers Chronic inflammatory cells, giant cells fibroblasts and Oreign body wrapped calcifications seen around the calcification Gross appearance Polycystic or solid pale yellow or grayish brown lesions Polycystic or solid pale yellow or grayish brown lesions -
[1] Kannan S, Ravikumar L, Mahadevan S, et al. Tumoral calcinosis with vitamin D deficiency[J]. Saudi J Kidney Dis Transplant, 2008, 19(6): 960-3. [2] Mohamed S, Jong-Hun J, Weon-Yoo K. Tumoral calcinosis of the foot with unusual presentation in an 11-year-old boy: a case report and review of literature[J]. J Postgrad Med, 2007, 53(4): 247. doi: 10.4103/0022-3859.37513 [3] Jeong JJ, Ji JH, Shafi M, et al. Hallux valgus deformity of foot with tumoral calcinosis: an unusual presentation[J]. Foot Ankle Surg, 2014, 20(1): e15-8. doi: 10.1016/j.fas.2013.10.007 [4] Shah A, Miller CJ, Nast CC, et al. Severe vascular calcification and tumoral calcinosis in a family with hyperphosphatemia: a fibroblast growth factor 23 mutation identified by exome sequencing[J]. Nephrol Dial Transplant, 2014, 29(12): 2235-43. doi: 10.1093/ndt/gfu324 [5] Tharini GK, Prabhavathy D, Hema N, et al. Idiopathic sporadic tumoral calcinosis in an Indian girl[J]. Indian J Dermatol Venereol Leprol, 2011, 77(4): 536. [6] 李涤臣, 承泽龙, 章可仪, 等. 瘤样钙质沉着症7例临床病理分析[J]. 临床与实验病理学杂志, 1993, 9(S1): 47-8. https://www.cnki.com.cn/Article/CJFDTOTAL-LSBL1993S1026.htm [7] Savaci N, Avunduk MC, Tosun Z, et al. Hyperphosphatemic tumoral calcinosis[J]. Plast Reconstr Surg, 2000, 105(1): 162-5. doi: 10.1097/00006534-200001000-00027 [8] Smack D, Norton SA, Fitzpatrick JE. Proposal for a pathogenesisbased classification of tumoral calcinosis[J]. Int J Dermatol, 1996, 35(4): 265-71. doi: 10.1111/j.1365-4362.1996.tb02999.x [9] Topaz O, Indelman M, Chefetz I, et al. A deleterious mutation in SAMD9 causes normophosphatemic familial tumoral calcinosis [J]. Am J Hum Genet, 2006, 79(4): 759-64. doi: 10.1086/508069 [10] Chefetz I, Ben Amitai D, Browning S, et al. Normophosphatemic familial tumoral calcinosis is caused by deleterious mutations in SAMD9, encoding a TNF- α responsive protein[J]. J Investig Dermatol, 2008, 128(6): 1423-9. doi: 10.1038/sj.jid.5701203 [11] Farzan M, Farhoud AR. Tumoral calcinosis: what is the treatment? Report of two cases of different types and review of the literature [J]. Am J Orthop (Belle Mead NJ), 2011, 40(9): E170-6. [12] Ho BB, Bergwitz C. FGF23 signalling and physiology[J]. J Mol Endocrinol, 2021, 66(2): R23-R32. doi: 10.1530/JME-20-0178 [13] Garringer HJ, Fisher C, Larsson TE, et al. The role of mutant UDPN-acetyl-alpha-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis[J]. J Clin Endocrinol Metab, 2006, 91(10): 4037-42. doi: 10.1210/jc.2006-0305 [14] Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23[J]. Nature, 2006, 444(7120): 770-4. doi: 10.1038/nature05315 [15] Chakhtoura M, Ramnitz MS, Khoury N, et al. Hyperphosphatemic familial tumoral calcinosis secondary to fibroblast growth factor 23 (FGF23) mutation: a report of two affected families and review of the literature[J]. Osteoporos Int, 2018, 29(9): 1987-2009. doi: 10.1007/s00198-018-4574-x [16] Leung YY, Lai R. Tumoral calcinosis: a case report[J]. J Orthop Surg, 2011, 19(1): 108-12. doi: 10.1177/230949901101900125 [17] Duret MH. Tumours multiples et singulieres des bourses sereuses (endotheliomes, peutetre d'origine parasitaire)[J]. Bull Mem Soc Anat Paris, 1899, 74: 725-33. [18] Rafaelsen S, Johansson S, Ræder H, et al. Long- term clinical outcome and phenotypic variability in hyperphosphatemic familial tumoral calcinosis and hyperphosphatemic hyperostosis syndrome caused by a novel GALNT3 mutation; case report and review of the literature[J]. BMC Genet, 2014, 15(1): 1-9. doi: 10.1186/1471-2156-15-1 [19] Li Voon Chong SW, Kion SA, Cullen MJ. A report of familial hyperphosphataemia in an Irish family[J]. Ir J Med Sci, 1999, 168 (4): 262-4. doi: 10.1007/BF02944354 [20] McGrath E, Harney F, Kinsella F. An ocular presentation of familial tumoral calcinosis[J]. Case Rep, 2010, 2010(sep17 1): bcr0520103044. [21] Ramnitz MS, Gourh P, Goldbach-Mansky R, et al. Phenotypic and genotypic characterization and treatment of a cohort with familial tumoral calcinosis/hyperostosis-hyperphosphatemia syndrome[J]. J Bone & Mineral Res, 2016, 31(10): 1845-54. [22] Yamaguchi T, Sugimoto T, Imai Y, et al. Successful treatment of hyperphosphatemic tumoral calcinosis with long- term acetazolamide[J]. Bone, 1995, 16(4): S247-50. doi: 10.1016/8756-3282(95)00019-A [23] Boyce AM, Lee AE, Roszko KL, et al. Hyperphosphatemic tumoral calcinosis: pathogenesis, clinical presentation, and challenges in management[J]. Front Endocrinol, 2020, 11: 293. doi: 10.3389/fendo.2020.00293 [24] Ichikawa S, Baujat G, Seyahi A, et al. Clinical variability of familial tumoral calcinosis caused by novel GALNT3 mutations [J]. Am J Med Genet Pt A, 2010, 152A(4): 896-903. doi: 10.1002/ajmg.a.33337 [25] Döneray H, Özden A, Gürbüz K. The successful treatment of deep soft-tissue calcifications with topical sodium thiosulphate and acetazolamide in a boy with hyperphosphatemic familial tumoral calcinosis due to a novel mutation in FGF23[J]. Jcrpe, 2022, 14(2): 239-43. doi: 10.4274/jcrpe.galenos.2021.2020.0269 [26] Tiwari V, Goyal A, Nagar M, et al. Hyperphosphataemic tumoral calcinosis[J]. Lancet, 2019, 393(10167): 168. doi: 10.1016/S0140-6736(18)33045-9 -







 下载:
下载: