Imaging and pathological features of primary solitary fibrous tumors in abdomen and pelvis
-
摘要:
目的 对照病理分析腹盆部原发孤立性纤维瘤(SFT)的影像特征表现。 方法 回顾性分析12例经病理证实为腹盆部SFT患者的影像资料,其中4例行CT检查,2例行MRI检查,6例同时行CT及MRI检查,并与病理结果进行对照分析。 结果 SFT发生于腹腔4例,盆腔5例,腹股沟区1例,同时位于腹盆腔2例。肿块呈囊实性1例、实性11例,最大径6.3~20.0 cm。CT平扫肿块呈等/低密度,病灶内见钙化灶3例,出血灶1例。MRI平扫T1WI呈等低信号8例,T2WI/T2压脂呈均匀低信号1例,余7例均呈高低混杂信号,特征“阴阳图”4例,瘤内见流空血管影4例。增强扫描:12例均呈不均匀强化,10例呈“地图样”强化,2例呈花斑样强化,其中1例呈持续强化,1例呈渐进性强化,其余10例呈“快进慢出型”强化;6例瘤内见迂曲血管影,即“蚯蚓钻土”征。除坏死囊变区外,将8例行MRI检查的肿瘤实性成分分为两类:A类结节,T1WI等信号,T2WI/T2压脂低信号,增强检查无强化;B类结节,T1WI等低信号,T2WI/T2压脂高信号,增强检查呈轻-中度/显著强化,5例肿块为两类结节共存,3例为B类结节。病理示:5例肿瘤细胞见异型性及核分裂像增多,为非典型或恶性SFT。12例SFT的免疫组化标记物中,CD34阳性率100%,STAT6及Bcl-2阳性率91.7%,Vimentin阳性率83.3%,CD99阳性率75%。 结论 腹盆部SFT存在特征性影像表现,且肿块内部不同影像特征与病理组织成分相对应。肿块直径 > 10 cm或瘤内出现不规则钙化灶时,应警惕肿瘤恶变可能。结合免疫组化标记物对该疾病的诊断及鉴别诊断具有重要意义。 Abstract:Objective To analyze the imaging features of primary solitary fibrous tumors (SFT) in the abdomen and pelvis, according to the pathology. Methods We retrospectively analyzed the imaging data of 12 cases with pathologically confirmed abdominal and pelvic SFT, 4 cases underwent CT examination, and 2 cases underwent MRI examination. 6 cases underwent CT and MRI examination at the same time, and the pathological results were compared with the analysis of the pathological results. Results On plain CT scan, the mass showed equal / low density. There were 3 cases of calcification and 1 case of hemorrhage. On MRI plain scan, 8 cases showed isohypotension on T1WI, 1 case showed uniform hypotension on T2WI / T2 lipid pressure, the other 7 cases showed high and low mixed signals, 4 cases showed "Yin-Yang" pattern, 4 cases showed flow empty vessels in tumor. Enhanced scanning: 12 cases were inhomogeneous enhancement, 10 cases were "map like" enhancement, 2 cases were piebald like enhancement, of which 1 case was continuous enhancement, 1 case was gradual enhancement, and the remaining 10 cases were "fast in and slow out" enhancement. Tortuous blood vessels were seen in the tumors in 6 cases, namely "earthworm digging into the earth" sign. Except for necrotic cystic areas, the solid components of the tumor examined by MRI were divided into two categories: Type A nodules, T1WI isointense, T2WI / T2 hypopolipid signal, no enhancement on enhanced examination; Type B nodules, low signal intensity on T1WI and high signal intensity on T2WI / T2 lipid pressure, showed mild to moderate / significant enhancement on enhanced examination. 5 cases of mass were 2 types of nodules coexisting, and 3 cases were type B nodules. Pathology showed that 5 cases of tumor cells showed atypical or malignant SFT. The positive rate of CD34 was 100%, STAT6 and Bcl-2 were 91.7%, Vimentin was 83.3%, and CD99 was 75%. Conclusion There are characteristic imaging features in abdominal and pelvic SFT, and different imaging features in the mass correspond to the pathological tissue components. When the diameter of the mass > 10 cm or the irregular calcium focus in the tumor, we should be vigilant against the possibility of malignant transformation of the tumor. Combined with immunohistochemical markers is of great significance in the diagnosis and differential diagnosis of the disease. -
图 1 男性,56岁,右侧小肠系膜区孤立性纤维瘤
Figure 1. Male, 56 years old, solitary fibroma in the right mesenteric region. A-B: moderate progressive strengthening in the arterial phase and venous phase, patchy unreinforced area in the tumor, characteristic "map-like" enhancement; C: The pathology shows that spindle-shaped tumor cells and collagen fibers are intertwined, and multiple thickened, thick-walled blood vessels are seen in the interstitium (HE staining, ×100).
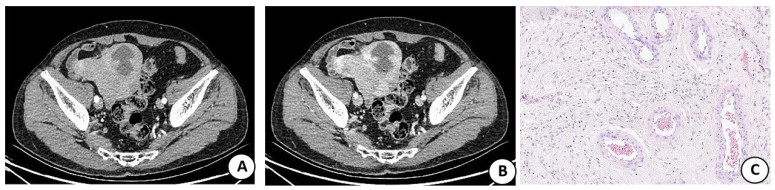
图 2 女,65岁,左侧腹膜后孤立性纤维瘤
Figure 2. Female, 65 years old, left retroperitoneal solitary fibroma. A: CT enhances the uneven strengthening of the arterial stage, and the tortuous vascular shadow (white short arrow) was seen in the tumor, showing "earthworm drilling sign"; B: T1WI showed an isolow signal; C: T2WI lipid compression showed high and low mixed signals, see flaky low signal region (white short arrow); D: MRI-enhanced arterial phase was spotted strengthening, and tortuous vascular shadows (white long arrows) could be seen in the tumor, and the flaky unreinforced area (white short arrows) corresponded to the T2WI low signal area, which was a class A nodule; E: B nodules corresponded to pathological images, spindle tumor cells were dense and disorganized (HE staining, ×200); F: Class A nodules corresponded to pathological images, mainly distributed by dense mature collagen fibers, tumor cells were sparse, and blood vessels were rare(HE staining, ×200).
图 3 男,55岁,盆腔孤立性纤维瘤
Figure 3. Male, 55 years old, pelvic solitary fibroma. A: T2WI showed uneven signal of the lesion, and the vascular shadow (white short arrow) was seen in the tumor; B: MRI contrast examination showed uneven "map" reinforcement; C: Pathology shows dense and alternating distribution of spindle-shaped tumor cells, collagen fibers and vascular distribution in the interstitium (HE staining×100).
表 1 SFT的免疫组化表现
Table 1. Immunohistochemical manifestations of SFT (n)
Position CD34 CD99 STAT6 Bcl-2 Vimentin SMA S-100 (+/-) (+/-) (+/-) (+/-) (+/-) (+/-) (+/-) Mesentery (n=2) 2/0 2/0 2/0 1/1 2/0 0/2 0/2 Retroperitoneal (n=2) 2/0 2/0 2/0 2/0 1/1 0/2 0/2 Pelvic cavity(n=5) 5/0 4/1 5/0 5/0 4/1 0/5 0/5 Groin (n=1) 1/0 0/1 1/0 1/0 1/0 0/1 0/1 Abdominal-pelvic cavity (n=2) 2/0 1/1 2/0 2/0 2/0 0/2 0/2 Total (n=12) 12/0 9/3 11/1 11/1 10/2 0/12 0/12 SFT: Solitary fibrous tumor. -
[1] Wang YX, Zhong Y, Fan SS, et al. Solitary fibrous tumors of the lung: a clinicopathological analysis of 52 cases[J]. Curr Oncol, 2023, 30(2): 1784-93. doi: 10.3390/curroncol30020138 [2] Abraham BT, Balaji P, Lee JW, et al. Malignant extrapleural solitary fibrous tumor[J]. Cureus, 2023, 15(8): e43750. [3] Huang AQ, Su MY, Jing YL, et al. Orbital primary solitary fibrous tumor: a proposed recurrence risk prediction model based on 92 cases[J]. Hum Pathol, 2023, 137: 85-93. doi: 10.1016/j.humpath.2023.04.018 [4] Nhung TH, Minh VL, Lam NL, et al. Malignant intracranial solitary fibrous tumor: a case report and literature review[J]. Radiol Case Rep, 2023, 18(5): 2014-8. doi: 10.1016/j.radcr.2023.02.064 [5] Li QH, Zhang CM, Li ZJ. Delayed pulmonary metastasis and recurrence of intracranial malignant solitary fibrous tumor/hemangiopericytoma: case report and literature review[J]. Oncol Lett, 2022, 24(2): 255. doi: 10.3892/ol.2022.13375 [6] Sexton G, McLoughlin J, Burke L, et al. Solitary fibrous tumour of mediastinum: an often asymptomatic neoplasm[J]. BMJ Case Rep, 2021, 14(8): e241223. doi: 10.1136/bcr-2020-241223 [7] 张宇泽, 杨云竣, 陈静勿, 等. 血管瘤型脑膜瘤与颅内孤立性纤维瘤的MRI征象分析[J]. 临床放射学杂志, 2022, 41(11): 2010-4. https://www.cnki.com.cn/Article/CJFDTOTAL-LCFS202211005.htm [8] Jeong J, Jin SY. Extrapleural malignant solitary fibrous tumor presenting as invasive bilateral paravertebral tumors: a case report[J]. J Korean Soc Radiol, 2023, 84(1): 304. doi: 10.3348/jksr.2022.0071 [9] 贡其星, 范钦和. 2020版WHO软组织肿瘤分类解读(二)[J]. 中华病理学杂志, 2021, 50(4): 314-8. [10] Dudde F, Giese M, Henkel KO. Metastasis of a solitary fibrous tumor in the mandible: a case report[J]. Cancer Diagn Progn, 2022, 3(1): 107-14. doi: 10.21873/cdp.10187 [11] Mohammed T, Ozcan G, Siddique AS, et al. Doege-potter syndrome with a benign solitary fibrous tumor: a case report and literature review[J]. Case Rep Oncol, 2021, 14(1): 470-6. doi: 10.1159/000512823 [12] Badawy M, Nada A, Crim J, et al. Solitary fibrous tumors: Clinical and imaging features from head to toe[J]. Eur J Radiol, 2022, 146: 110053. doi: 10.1016/j.ejrad.2021.110053 [13] Mack T, Purgina B. Updates in pathology for retroperitoneal soft tissue sarcoma[J]. Curr Oncol, 2022, 29(9): 6400-18. doi: 10.3390/curroncol29090504 [14] Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors[J]. Cancer, 2002, 94(4): 1057-68. doi: 10.1002/cncr.10328 [15] Nitta T, Kimura K, Tominaga T, et al. Malignant solitary fibrous tumor of the breast[J]. Breast J, 2021, 27(4): 391-3. doi: 10.1111/tbj.14175 [16] 陈明, 查云飞, 王艳艳, 等. 腹盆腔孤立性纤维瘤的影像学表现并文献复习[J]. 实用放射学杂志, 2019, 35(3): 494-7. [17] Ma ZL, Shi HB, Fang XS, et al. Variable solitary fibrous tumor locations[J]. Medicine, 2016, 95(13): e3031. doi: 10.1097/MD.0000000000003031 [18] 梅磊磊, 聂蕾, 唐文英, 等. 孤立性纤维瘤的影像表现及临床病理特征[J]. 放射学实践, 2022, 37(5): 566-70. https://www.cnki.com.cn/Article/CJFDTOTAL-FSXS202205007.htm [19] Ahmed TM, Blanco A, Weisberg EM, et al. CT of retroperitoneal solitary fibrous tumor[J]. Radiol Case Rep, 2023, 18(6): 2241-4. doi: 10.1016/j.radcr.2023.03.041 [20] 周建功, 马小龙, 汪建华, 等. 盆腔孤立性纤维瘤的MR特征及与病理结果对照[J]. 中华放射学杂志, 2014, 48(1): 47-51. [21] 李守红, 蒋沫轩, 邹先进, 等. 腹盆部孤立性纤维瘤的影像表现及病理对照[J]. 实用放射学杂志, 2021, 37(7): 1118-21, 1168. doi: 10.3969/j.issn.1002-1671.2021.07.017 [22] 陈婷婷, 魏明翔, 朱艳, 等. ADC直方图分析在胃肠道间质瘤分级中的应用[J]. 磁共振成像, 2021, 12(2): 79-82. https://www.cnki.com.cn/Article/CJFDTOTAL-CGZC202102018.htm [23] Okuda M, Yoshida K, Kobayashi S, et al. Desmoid-type fibromatosis: imaging features and course[J]. Skeletal Radiol, 2023, 52(7): 1293-303. [24] Lacuna K, Bose S, Ingham M, et al. Therapeutic advances in leiomyosarcoma[J]. Front Oncol, 2023, 13: 1149106. [25] Maçaneiro Junior CH, Baptista AM, Camargo OPD, et al. Undifferentiated pleomorphic sarcoma: prognostic factors in 42 extremity cases[J]. Acta Ortop Bras, 2023, 31(2): e265942. [26] Rückert JC, Elsner A, Andreas MN. Mediastinale tumore[J]. Zentralbl Chir, 2022, 147(1): 99-120. -







 下载:
下载: