The liver targeting of Mn-BnO-TyrEDTA and its preliminary application in a mouse liver cancer model
-
摘要:
目的 评价锰苄基酪氨酸乙二胺四乙酸(Mn-BnO-TyrEDTA)MRI对比剂的肝靶向性及其在小鼠肝癌模型上的初步运用。 方法 经尾静脉注射相同剂量的Mn-BnO-TyrEDTA和钆塞酸二钠(Gd-EOB-DTPA),并对小鼠进行MRI动态增强扫描,持续1 h,以监测对比剂的分布和清除情况。同时在小鼠肝脏上注射肝癌细胞H22(1×105/30 μL)和基质胶(25 μL)的混合液以建立肝癌模型,并对上述模型进行MRI。将钆喷葡胺作为对照组。 结果 注射Mn-BnO-TyrEDTA 1 min后肝脏开始强化,信号强度在3 min时达到峰值并持续至30 min;1 min后血液和肾脏信号强度开始明显降低,3 min后可见对比剂进入膀胱。通过比较3 min归一化信噪比和对比噪声比,判断两种对比剂肝靶向的强弱:Mn-BnO-TyrEDTA的归一化信噪比和对比噪声比分别为3.07±0.38和11±4.0,Gd-EOB-DTPA的归一化信噪比和对比噪声比分别为2.06±0.11和4.4±0.4,差异有统计学意义(P<0.05)。与正常肝实质比较,小鼠肝癌模型的肝内病灶呈明显低信号。 结论 Mn-BnO-TyrEDTA具有肝靶向性,并且经肝脏和肾脏双重途径清除,其在1~5 min的肝靶向性明显强于Gd-EOB-DTPA。小鼠肝癌模型的肝内病灶与周围正常肝实质具有较好的信号差异。 -
关键词:
- 锰苄基酪氨酸乙二胺四乙酸 /
- 肝靶向MRI对比剂 /
- 钆塞酸二钠 /
- 钆喷葡胺 /
- 肝癌模型
Abstract:Objective To evaluate the liver targeting of manganese benzyltyrosine ethylenediamine tetraacetic acid (Mn-BnO-TyrEDTA) MRI contrast agent and its preliminary application in mouse liver cancer model. Methods Mice were given by the same dose of Mn-BnO-TyrEDTA and disodium gadolinium serate (Gd-EOB-DTPA) via a caudal vein, and dynamic enhanced MRI scans were performed for 1 h to monitor the distribution and clearance of contrast agents. Simultaneously injecting liver cancer cell H22 (1×105/30 μL) into mouse liver and matrix adhesive (25 μL) using a mixed solution to establish a liver cancer model and perform MRI imaging. Gd-DTPA was used as the control group. Results After injecting Mn-BnO-TyrEDTA for 1 min, the liver began to strengthen, and the signal intensity reached its peak at 3 min and continued until 30 min; After 1 min, the blood and kidney signal intensity began to significantly decrease, and after 3 min, the contrast agent entered the bladder. By comparing the normalized signal‑to‑noise ratio and contrast‑to‑noise ratio for 3 min, it was determined that the liver targeting values of the two contrast agents were 3.07±0.38 and 11±4.0 for Mn-BnO-TyrEDTA and 2.06±0.11 and 4.4±0.4 for Gd-EOB-DTPA, respectively, with statistical differences (P<0.05). Compared with normal liver parenchyma, the intrahepatic lesions in the mouse liver cancer model showed significantly low signal intensity. Conclusion Mn-BnO-TyrEDTA has liver targeting, and it is cleared through both liver and kidney pathways. Its liver targeting is significantly stronger than Gd-EOB-DTPA at 1-5 min. There is a good signal difference between the intrahepatic lesions of the mouse liver cancer model and the surrounding normal liver parenchyma. -
图 2 注射相同剂量的Mn-BnO-TyrEDTA与Gd-EOB-DTPA以后,小鼠肝脏、血液、肾脏的MRI nSNR-Time曲线图和∆CNR柱状图
Figure 2. Comparison of MRI nSNR-Time curves and ∆CNR histograms of mouse liver, blood, and kidneys after injection of the same dose of Mn-BnO-TyrEDTA and Gd-EOB-DTPA. A: MRI nSNR-Time curve of mouse liver; B: MRI nSNR-Time curve of mouse blood; C: MRI nSNR-Time curve of the kidney; D: Histogram of ∆CNR in the liver of mice in the first 5 min.
表 1 注射相同剂量的两种对比剂在小鼠肝脏中的MRI信号强度
Table 1. MRI signal intensity of two contrast agents injected with the same dose in mouse liver (n=5)
Injection time SI Mn-BnO-TyrEDTA Gd-EOB-DTPA t P 3 min nSNR 3.07±0.38 2.06±0.11 4.354 0.004 CNR 11±4.0 4.4±0.4 2.79 0.03 SI: Signal intensity. -
[1] Wahsner J, Gale EM, Rodríguez-Rodríguez A, et al. Chemistry of MRI contrast agents: current challenges and new frontiers[J]. Chem Rev, 2019, 119(2): 957-1057. doi: 10.1021/acs.chemrev.8b00363 [2] Kim SY. Diagnosis of hepatocellular carcinoma: which MRI contrast agent? Which diagnostic criteria?[J]. Clin Mol Hepatol, 2020, 26(3): 309-11. doi: 10.3350/cmh.2020.0061 [3] Kojiro M. Focus on dysplastic nodules and early hepatocellular carcinoma: an Eastern point of view[J]. Liver Transplant, 2004, 10(S2): S3-8. doi: 10.1002/lt.20042 [4] Jhaveri K, Cleary S, Audet P, et al. Consensus statements from a multidisciplinary expert panel on the utilization and application of a liver-specific MRI contrast agent (gadoxetic acid)[J]. Am J Roentgenol, 2015, 204(3): 498-509. doi: 10.2214/AJR.13.12399 [5] Pastor CM, Planchamp C, Pochon S, et al. Kinetics of gadobenate dimeglumine in isolated perfused rat liver: MR imaging evaluation[J]. Radiology, 2003, 229(1): 119-25. doi: 10.1148/radiol.2291020726 [6] Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based contrast agents by the food and drug administration[J]. Radiology, 2012, 265(1): 248-53. doi: 10.1148/radiol.12112783 [7] Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging[J]. J Am Soc Nephrol, 2006, 17(9): 2359-62. doi: 10.1681/ASN.2006060601 [8] Semelka RC, Commander CW, Jay M, et al. Presumed gadolinium toxicity in subjects with normal renal function[J]. Invest Radiol, 2016, 51(10): 661-5. doi: 10.1097/RLI.0000000000000318 [9] McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities[J]. Radiology, 2017, 285(2): 546-54. doi: 10.1148/radiol.2017161595 [10] McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging[J]. Radiology, 2015, 275(3): 772-82. doi: 10.1148/radiol.15150025 [11] Kralik SF, Singhal KK, Frank MS, et al. Evaluation of gadolinium deposition in the brain after MR arthrography[J]. Am J Roentgenol, 2018, 211(5): 1063-7. doi: 10.2214/AJR.18.19833 [12] Kiviniemi A, Gardberg M, Ek P, et al. Gadolinium retention in gliomas and adjacent normal brain tissue: association with tumor contrast enhancement and linear/macrocyclic agents[J]. Neuroradiology, 2019, 61(5): 535-44. doi: 10.1007/s00234-019-02172-6 [13] Aschner J L, Aschner M. Nutritional aspects of Manganese homeostasis[J]. Mol Aspects Med, 2005, 26(4/5): 353-62. [14] Jørgensen JT, Rief M, Brismar TB, et al. A new Manganese-based oral contrast agent (CMC-001) for liver MRI: pharmacological and pharmaceutical aspects[J]. Acta Radiol, 2012, 53(7): 707-13. doi: 10.1258/ar.2012.120034 [15] Santamaria AB, Sulsky SI. Risk assessment of an essential element: Manganese[J]. J Toxicol Environ Health A, 2010, 73(2/3): 128-55. [16] Thomsen HS, Svendsen O, Klastrup S. Increased Manganese concentration in the liver after oral intake1[J]. Acad Radiol, 2004, 11(1): 38-44. doi: 10.1016/S1076-6332(03)00571-3 [17] Toft KG, Hustvedt SO, Grant D, et al. Metabolism and pharmacokinetics of MnDPDP in man[J]. Acta Radiol, 1997, 38(5): 677-89. doi: 10.1080/02841859709172400 [18] Young SW, Simpson BB, Ratner AV, et al. MRI measurement of hepatocyte toxicity using the new MRI contrast agent Manganese dipyridoxal diphosphate, a Manganese/pyridoxal 5-phosphate chelate[J]. Magn Reson Med, 1989, 10(1): 1-13. doi: 10.1002/mrm.1910100102 [19] Lim KO, Stark DD, Leese PT, et al. Hepatobiliary MR imaging: first human experience with MnDPDP[J]. Radiology, 1991, 178(1): 79-82. doi: 10.1148/radiology.178.1.1898539 [20] Pan D, Schmieder AH, Wickline SA, et al. Manganese-based MRI contrast agents: past, present and future[J]. Tetrahedron, 2011, 67(44): 8431-44. doi: 10.1016/j.tet.2011.07.076 [21] Gale EM, Wey HY, Ramsay I, et al. A Manganese-based alternative to gadolinium: contrast-enhanced MR angiography, excretion, pharmacokinetics, and metabolism[J]. Radiology, 2018, 286(3): 865-72. doi: 10.1148/radiol.2017170977 [22] Gale EM, Atanasova IP, Blasi F, et al. A Manganese alternative to gadolinium for MRI contrast[J]. J Am Chem Soc, 2015, 137(49): 15548-57. doi: 10.1021/jacs.5b10748 [23] Troughton JS, Greenfield MT, Greenwood JM, et al. Synthesis and evaluation of a high relaxivity Manganese(Ⅱ)-based MRI contrast agent[J]. Inorg Chem, 2004, 43(20): 6313-23. doi: 10.1021/ic049559g [24] Tan MQ, Ye Z, Jeong EK, et al. Synthesis and evaluation of nanoglobular macrocyclic Mn(Ⅱ) chelate conjugates as non-gadolinium(Ⅲ) MRI contrast agents[J]. Bioconjug Chem, 2011, 22(5): 931-7. doi: 10.1021/bc100573t [25] Bollow M, Taupitz M, Hamm B, et al. Gadolinium-ethoxybenzyl-DTPA as a hepatobiliary contrast agent for use in MR cholangiography: results of an in vivo phase-Ⅰ clinical evaluation[J]. Eur Radiol, 1997, 7(1): 126-32. doi: 10.1007/s003300050125 [26] Caravan P, Ellison JJ, McMurry TJ, et al. Gadolinium(Ⅲ) chelates as MRI contrast agents: structure, dynamics, and applications[J]. Chem Rev, 1999, 99(9): 2293-352. doi: 10.1021/cr980440x [27] Peng JF, Yang GP, Huang ZJ. Vitamin D deficiency impacts exposure and response of pravastatin in male rats by altering hepatic OATPs[J]. Front Pharmacol, 2022, 13: 841954. doi: 10.3389/fphar.2022.841954 [28] Elizabeth C, Zoe W, Swanson M, et al. Single-cell image analysis reveals over-expression of organic anion transporting polypeptides (OATPs) in human glioblastoma tissue[J]. Neuro Oncol Adv, 2022, 4(1). [29] Ali Y, Shams T, Wang K, et al. The involvement of human organic anion transporting polypeptides (OATPs) in drug-herb/food interactions[J]. Chin Med, 2020, 15(1): 1-10. doi: 10.1186/s13020-019-0281-6 [30] Clement O, Muhler A, Vexler V, et al. Gadolinium-ethoxybenzyl-DTPA, a new liver-specific magnetic resonance contrast agent: kinetic and enhancement patterns in normal and cholestatic rats[J]. Investig Radiol, 1992, 27(8): 612-9. doi: 10.1097/00004424-199208000-00010 [31] Montfoort JV, Stieger B, Meijer D, et al. Hepatic uptake of the magnetic resonance imaging contrast agent gadoxetate by the organic anion transporting polypeptide Oatp1[J]. J Pharmacol Exp Ther, 1999, 290(1): 153-7. [32] Chen KY, Li P, Zhu CR, et al. Mn(Ⅱ) complex of lipophilic group-modified ethylenediaminetetraacetic acid (EDTA) as a new hepatobiliary MRI contrast agent[J]. J Med Chem, 2021, 64(13): 9182-92. doi: 10.1021/acs.jmedchem.1c00393 -
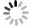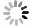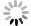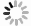






 下载:
下载: