Efficacy of sintilimab combined with chemotherapy neoadjuvant in the treatment of stage Ⅲ non-small cell lung cancer
-
摘要:
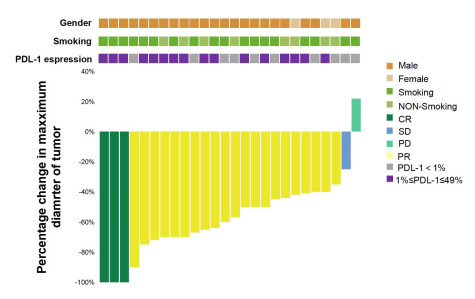
目的 评价信迪利单抗联合化疗新辅助治疗Ⅲ期非小细胞肺癌的近期疗效和安全性。 方法 分析2021年1月~2023年7月我院26例使用信迪利单抗联合化疗新辅助治疗Ⅲ期非小细胞肺癌患者的临床资料,评估患者影像学和病理学效果并观察免疫联合化疗新辅助治疗的安全性。 结果 23例患者完成根治性手术治疗,手术切除率88.5%,22例R0切除,R0切除率95.7%。影像学结果客观缓解率为92.3%(完全缓解率为11.5%,部分缓解为80.8%);疾病控制率为96.1%,病理完全缓解率为26.1%,主要病理缓解率为60.9%。常见不良反应:白细胞下降(42.3%)、外周神经毒性(46.2%)、消化道不良反应(23.1%)、甲状腺功能异常(11.5%),3级以上不良反应发生率约为7.7%,未发生严重免疫相关不良反应。 结论 信迪利单抗联合化疗新辅助治疗Ⅲ期非小细胞肺癌的疗效显著,R0切除率高,病理缓解率高,安全性可耐受。 Abstract:Objective To evaluate the short-term efficacy and safety of sintilimab combined with chemotherapy neoadjuvant in the treatment of stage Ⅲ non-small cell lung cancer. Methods The clinical data of 26 patients with stage Ⅲ non-small cell lung cancer treated with Sintilimab combined with chemotherapy neoadjuvant therapy in our hospital from January 2021 to July 2023 were analyzed. The imaging and pathological effects of the patients were evaluated. The safety of immunotherapy combined with chemotherapy neoadjuvant therapy was observed. Results Twenty-three patients completed radical surgery with a surgical resection rate of 88.5%, and 22 patients underwent R0 resection with a R0 resection rate of 95.7%. Objective response rate of imaging results was 92.3% (complete response rate was 11.5%, partial response rate was 80.8%). The disease control rate was 96.1%, the pathological complete response rate was 26.1%, and the main pathological response rate was 60.9%. Common adverse reactions: The incidence of leukopenia was 42.3%, peripheral neurotoxicity was 46.2%, gastrointestinal adverse reactions were 23.1%, thyroid dysfunction was 11.5%, and the incidence of grade 3 or above adverse reactions was about 7.7%. No serious immune related adverse reactions had occurred. Conclusion The combination of sintilimab and neoadjuvant chemotherapy has a significant therapeutic effect on stage Ⅲ non-small cell lung cancer, with a high R0 resection rate, high pathological response rate, and tolerable safety. -
Key words:
- sintilimab /
- chemotherapy /
- neoadjuvant therapy /
- stage Ⅲ non-small cell lung cancer /
- efficacy
-
图 2 1例肺鳞状细胞癌患者影像和病理评估(完全缓解)
Figure 2. Imaging and pathological evaluation of 1 patient with lung squamous cell carcinoma (complete response). A-B: Enhanced chest CT before neoadjuvant chemotherapy revealed a 4.4 cm mass in the upper lobe of the right lung invading the right upper pulmonary artery; C: Complete imaging response after 3 cycles of neoadjuvant chemotherapy; D: Tracheal pathology of squamous cell carcinoma before neoadjuvant chemotherapy (HE staining, ×10); E: Pathological complete response after 3 cycles of neoadjuvant chemotherapy. Extensive inflammatory cell infiltration, foam macrophages, cholesterol crevices, collagen fiber formation, and neovasculation (HE staining, ×10).
图 3 1例肺腺癌患者影像和病理评估
Figure 3. Imaging and pathological evaluation of 1 patient with lung adenocarcinoma (complete response). A: Contrast-enhanced CT before neoadjuvant chemotherapy revealed a 3.5 cm mass in the upper lobe of the right lung surrounding the right upper pulmonary artery; B: Complete remission after 3 cycles of neoadjuvant chemotherapy; C: Tracheal pathology before neoadjuvant chemotherapy showed adenocarcinoma (HE staining, ×10); D: Pathological complete response after 3 cycles of neoadjuvant chemotherapy. Extensive inflammatory cell infiltration, foam macrophages, cholesterol crevices, collagen fiber formation, and neovasculation (HE staining, ×10).
图 4 1例肺鳞状细胞癌患者影像和病理评估(主要病理缓解)
Figure 4. Imaging and pathological evaluation of 1 patient with lung squamous cell (carcinomamajor pathological remission). A: Enhanced chest CT before neoadjuvant chemotherapy revealed a 6.0 cm mass in the upper lobe of the left lung, mediastinal lymph node enlargement; B: Partial remission after 3 cycles of neoadjuvant chemotherapy; C: Pathological findings after 3 cycles of neoadjuvant chemotherapy showed major pathological remission, and the proportion of residual tumor cells ≤10% (HE staining, ×10).
表 1 26例患者一般资料
Table 1. Clinical data of the 26 patients [n(%)]
Characteristics All patients (n=26) Gender Male 23(88.5) Female 3(11.5) Age (year) < 60 5(19.2) ≥60 21(80.8) Smoking status Yes 17(65.4) No 9(34.6) Tumor site Upper lobe of right lung 11(42.3) Middle lobe of right lung 2(7.70) Upper lobe of left lung 8(30.8) Inferior lobe of left lung 5(19.2) Pathological type Squamous cell carcinoma 16(61.5) Adenocarcinoma 8(30.8) Poorly differentiated carcinoma 1(3.85) Invasive carcinoma 1(3.85) Clinical disease stage ⅢA 14(53.8) ⅢB 10(38.5) ⅢC 2(7.7) ECOG 0-1 11(42.3) 2 15(57.7) Expression level of PD-L1 < 1% 10(38.5) 1%-49% 16(61.5) ECOG: Eastern cooperative oncology group. 表 2 23例患者的手术资料
Table 2. Surgical data of 23 patients [n(%)]
Characteristics All patients (n=23) Degree of excision R0 excision 22(95.7) R1 excision 1(4.30) Surgical procedure Lobectomy 20(87.1) Compound lobectomy 1(4.30) Complete resection of one lung 2(8.60) Mode of operation Thoracoscope 19(87.1) Open the chest 2(8.60) Conversion thoracotomy 1(4.30) Common surgical complications Atrial fibrillation 5(21.7) Mild pneumonia 8(34.9) Cardiac insufficiency 2(8.60) Bronchopleural fistula 1(4.30) Pathological remission Complete remission 6(26.1) Major pathological remission 14(60.9) -
[1] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020[J]. CA A Cancer J Clin, 2020, 70(1): 7-30. doi: 10.3322/caac.21590 [2] Men Y, Wang L, Zhang Y, et al. Trends of postoperative radiotherapy for completely resected non-small cell lung cancer in China: a hospital-based multicenter 10-year (2005-2014) retrospective clinical epidemiological study[J]. Front Oncol, 2019, 9: 786. doi: 10.3389/fonc.2019.00786 [3] Zhang Q, Fu XL, Cai XW, et al. Phenotypic analysis of failure in patients with complete resected stage ⅢA(N2) non-small cell lung cancer[J]. Chin J Oncol, 2017, 27(5): 383-8. [4] 裴斯宁, 于洪. Ⅲ期非小细胞肺癌术前新辅助治疗现状及进展[J]. 现代肿瘤医学, 2023, 31(2): 387-92. doi: 10.3969/j.issn.1672-4992.2023.02.038 [5] Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer[J]. N Engl J Med, 2018, 378(21): 1976-86. doi: 10.1056/NEJMoa1716078 [6] Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial[J]. Nat Med, 2021, 27(3): 504-14. doi: 10.1038/s41591-020-01224-2 [7] Provencio M, Nadal E, Insa A, et al. Faculty Opinions recommendation of Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial[J]. Lancet Oncol, 2020, 21(11): 1413-22. doi: 10.1016/S1470-2045(20)30453-8 [8] 刘雨桃, 高禹舜, 毛友生, 等. 程序性细胞死亡蛋白1单抗联合化疗在Ⅱ期和Ⅲ期非小细胞肺癌术前新辅助治疗中的疗效和安全性[J]. 中华肿瘤杂志, 2020, 42(6): 480-5. doi: 10.3760/cma.j.cn112152-20200213-00087 [9] NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data[J]. Lancet, 2014, 383(9928): 1561-71. doi: 10.1016/S0140-6736(13)62159-5 [10] Melero I, Beraondo P, Rodriguez-ruiz ME, et al. Making the most of cancer surgery with neoadjuvant immunotherapy[J]. Cancer Discov, 2016, 6(12): 1312-4. doi: 10.1158/2159-8290.CD-16-1109 [11] Keung EZ, Ukponmwan EU, Cogdill AP, et al. The rationale and emerging use of neoadjuvant immune checkpoint blockade for solid malignancies[J]. Ann Surg Oncol, 2018, 25(7): 1814-27. doi: 10.1245/s10434-018-6379-8 [12] Pall G. Neoadjuvant immunotherapy in nonsmall cell lung cancer[J]. Curr Opin Oncol, 2020, 33(1): 59-63. [13] Fournel L, Wu ZR, Stadler N, et al. Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer[J]. Cancer Lett, 2019, 464: 5-14. doi: 10.1016/j.canlet.2019.08.005 [14] Rothschild SI, Zippelius A, Prince SS, et al. 126TiP SAKK 16/14: Anti-PD-L1 antibody durvalumab (MEDI4736) in addition to neoadjuvant chemotherapy in patients with stage ⅢA(N2) non-small cell lung cancer (NSCLC): a multicenter single-arm phase Ⅱ trial[J]. J Thorac Oncol, 2018, 13(4): S70. [15] Travis WD, Dacic S, Wistuba I, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy[J]. J Thorac Oncol, 2020, 15(5): 709-40. doi: 10.1016/j.jtho.2020.01.005 [16] Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial[J]. Lancet Oncol, 2020, 21(6): 786-95. doi: 10.1016/S1470-2045(20)30140-6 [17] 黄焰, 张莉萍, 侯立坤, 等. 非小细胞肺癌新辅助治疗后手术切除标本的病理评估[J]. 中华病理学杂志, 2021, 50(7): 773-8. doi: 10.3760/cma.j.cn112151-20201224-00962 [18] Yamane Y, Ishii G, Goto K, et al. A novel histopathological evaluation method predicting the outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor[J]. J Thorac Oncol, 2010, 5(1): 49-55. doi: 10.1097/JTO.0b013e3181c0a1f8 [19] Gao SG, Li N, Gao SY, et al. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC[J]. J Thorac Oncol, 2020, 15(5): 816-26. doi: 10.1016/j.jtho.2020.01.017 [20] Eichhorn F, Klotz LV, Kriegsmann M, et al. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: first clinical experience[J]. Lung Cancer, 2021, 153: 150-7. doi: 10.1016/j.lungcan.2021.01.018 [21] Zhao ZR, Yang CP, Chen S, et al. Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage Ⅲ non-small-cell lung cancer[J]. Oncoimmunology, 2021, 10(1): 1996000. doi: 10.1080/2162402X.2021.1996000 [22] Wang SH, Yuan P, Mao BB, et al. Genomic features and tumor immune microenvironment alteration in NSCLC treated with neoadjuvant PD-1 blockade[J]. NPJ Precis Oncol, 2022, 6(1): 2. doi: 10.1038/s41698-021-00244-6 [23] Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer[J]. N Engl J Med, 2022, 386(21): 1973-85. doi: 10.1056/NEJMoa2202170 [24] Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer[J]. J Thorac Cardiovasc Surg, 2019, 158(1): 269-76. doi: 10.1016/j.jtcvs.2018.11.124 [25] Shao ME, Yao J, Wang YK, et al. Two vs three cycles of neoadjuvant sintilimab plus chemotherapy for resectable non-small-cell lung cancer: neoSCORE trial[J]. Signal Transduct Target Ther, 2023, 8(1): 146. doi: 10.1038/s41392-023-01355-1 [26] Jia XH, Xu H, Geng LY, et al. Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: a meta-analysis[J]. Lung Cancer, 2020, 147: 143-53. doi: 10.1016/j.lungcan.2020.07.001 -







 下载:
下载: