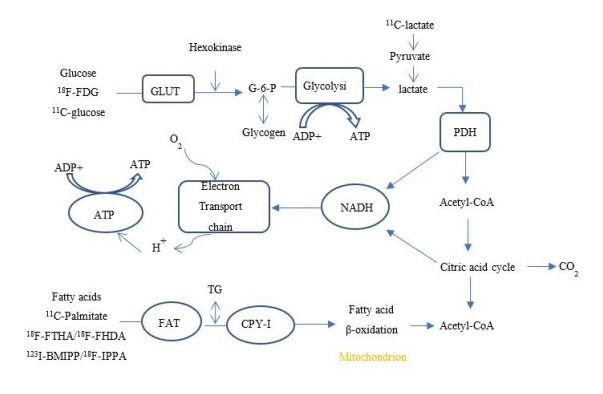
| [1] |
Beuthien-Baumann B, Sachpekidis C, Gnirs R, et al. Adapting imaging protocols for PET-CT and PET-MRI for immunotherapy monitoring[J]. Cancers (Basel), 2021, 13(23): 6019-32. doi: 10.3390/cancers13236019
|
| [2] |
Sadaghiani Mohammad S, Sara S, Rowe Steven P, et al. Cellular and molecular imaging with SPECT and PET in brain tumors[J]. Radiol Clin N Am, 2021, 59(3): 363-75. doi: 10.1016/j.rcl.2021.01.005
|
| [3] |
Gott M, Yang P, Kortz U, et al. A 224Ra-labeled polyoxopalladate as a putative radiopharmaceutical[J]. Chem Commun, 2019, 55 (53): 7631-4. doi: 10.1039/C9CC02587A
|
| [4] |
Kamani CH, Prior JO. Assessment of myocardial viability using a[15O]-water perfusion PET: towards a one-stop shop?[J]. J Nucl Cardiol, 2021, 28(4): 1281-3. doi: 10.1007/s12350-019-01838-1
|
| [5] |
Horsager J, Okkels N, Van Den Berge N, et al. In vivo vesicular acetylcholine transporter density in human peripheral organs: an 18FFEOBV PET/CT study[J]. EJNMMI Res, 2022, 12(1): 17-28. doi: 10.1186/s13550-022-00889-9
|
| [6] |
Effendi N, Mishiro K, Wakabayashi H, et al. Synthesis and evaluation of radiogallium-labeled long-chain fatty acid derivatives as myocardial metabolic imaging agents[J]. PLoS One, 2021, 16 (12): e0261226. doi: 10.1371/journal.pone.0261226
|
| [7] |
王雅枫, 周璐, 雷少青, 等. 缺血再灌注对大鼠心肌损伤及氧化应激的影响[J]. 中华实用诊断与治疗杂志, 2018, 32(12): 1157-9. https://www.cnki.com.cn/Article/CJFDTOTAL-HNZD201812004.htm
|
| [8] |
Gürel E, Smeele KM, Eerbeek O, et al. Ischemic preconditioning affects hexokinase activity and HKⅡ in different subcellular compartments throughout cardiac ischemia-reperfusion[J]. J Appl Physiol (1985), 2009, 106(6): 1909-16. doi: 10.1152/japplphysiol.90537.2008
|
| [9] |
Swinnen JV, Heemers H, Deboel L, et al. Stimulation of tumor-associated fatty acid synthase expression by growth factor activation of the sterol regulatory element-binding protein pathway[J]. Oncogene, 2000, 19(45): 5173-81. doi: 10.1038/sj.onc.1203889
|
| [10] |
Stendahl JC, Sinusas AJ. 11C-acetate PET: a powerful tool to analyze metabolic and functional changes in the heart related to alcohol consumption[J]. J Nucl Cardiol, 2022, 29(1): 289-92. doi: 10.1007/s12350-020-02268-0
|
| [11] |
Derlin T, Habermann CR, Lengyel Z, et al. Feasibility of 11C- acetate PET/CT for imaging of fatty acid synthesis in the atherosclerotic vessel wall[J]. J Nucl Med, 2011, 52(12): 1848-54. doi: 10.2967/jnumed.111.095869
|
| [12] |
Zeng H, He XC, Chen JX. Endothelial sirtuin 3 dictates glucose transport to cardiomyocyte and sensitizes pressure overload-induced heart failure[J]. J Am Heart Assoc, 2020, 9(11): e015895. doi: 10.1161/JAHA.120.015895
|
| [13] |
Lopaschuk GD, Karwi QG, Tian R, et al. Cardiac energy metabolism in heart failure[J]. Circ Res, 2021, 128(10): 1487-513. doi: 10.1161/CIRCRESAHA.121.318241
|
| [14] |
Brown SK, Sheikh AM, Guzik TJ. Cardiovascular Research at the frontier of biomedical science[J]. Cardiovasc Res, 2020, 116(7): e83-6. doi: 10.1093/cvr/cvaa119
|
| [15] |
Chan MY, Efthymios M, Tan SH, et al. Prioritizing candidates of post-myocardial infarction heart failure using plasma proteomics and single-cell transcriptomics[J]. Circulation, 2020, 142(15): 1408-21. doi: 10.1161/CIRCULATIONAHA.119.045158
|
| [16] |
Reivich M, Kuhl D, Wolf A, et al. Local cerebral glucose utilization measured with 18F-2-fluoro-2-deoxyglucose[J]. Int J Nucl Med Biol, 1978, 5(6): 290-7.
|
| [17] |
Cocker MS, Spence JD, Hammond R, et al. 18F-Fluorodeoxyglu-cose PET/CT imaging as a marker of carotid plaque inflammation: comparison to immunohistology and relationship to acuity of events[J]. Int J Cardiol, 2018, 271: 378-86. doi: 10.1016/j.ijcard.2018.05.057
|
| [18] |
Kircher M, Tran-Gia J, Kemmer L, et al. Imaging inflammation in atherosclerosis with CXCR4-directed 68Ga-pentixafor PET/CT: cor-relation with 18F-FDG PET/CT[J]. J Nucl Med, 2020, 61(5): 751-6. doi: 10.2967/jnumed.119.234484
|
| [19] |
Reivich M, Alavi A, Wolf A, et al. Glucose metabolic rate kinetic model parameter determination in humans: the lumped constants and rate constants for[18F]fluorodeoxyglucose and[11C]deoxyglu-cose[J]. J Cereb Blood Flow Metab, 1985, 5(2): 179-92. doi: 10.1038/jcbfm.1985.24
|
| [20] |
Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents[J]. Endocr Rev, 2019, 40(6): 1447-67. doi: 10.1210/er.2018-00141
|
| [21] |
Saotome M, Ikoma T, Hasan P, et al. Cardiac insulin resistance in heart failure: the role of mitochondrial dynamics[J]. Int J Mol Sci, 2019, 20(14): 3552. doi: 10.3390/ijms20143552
|
| [22] |
Berry JJ, Baker JA, Pieper KS, et al. The effect of metabolic milieu on cardiac PET imaging using fluorine-18-deoxyglucose and nitro-gen-13-ammonia in normal volunteers[J]. J Nucl Med, 1991, 32 (8): 1518-25.
|
| [23] |
Taylor M, Wallhaus TR, Degrado TR, et al. An evaluation of myo-cardial fatty acid and glucose uptake using PET with[18F]fluoro-6-thia-heptadecanoic acid and[18F]FDG in Patients with Congestive Heart Failure[J]. J Nucl Med, 2001, 42(1): 55-62.
|
| [24] |
Choi Y, Brunken RC, Hawkins RA, et al. Factors affecting myocar-dial 2-[F-18]fluoro-2-deoxy-d-glucose uptake in positron emission tomography studies of normal humans[J]. Eur J Nucl Med, 1993, 20(4): 308-18.
|
| [25] |
Rupp H, Rupp TP, Alter P, et al. Mechanisms involved in the differential reduction of omega-3 and omega-6 highly unsaturated fatty acids by structural heart disease resulting in ''HUFA deficienc''[J]. an J Physiol Pharmacol, 2012, 90(1): 55-73. doi: 10.1139/y11-101
|
| [26] |
Schwank-Xu C, Forsberg E, Bentinger M, et al. L-carnosine stimulation of coenzyme Q10 biosynthesis promotes improved mitochondrial function and decreases hepatic steatosis in diabetic conditions[J]. Antioxidants (Basel), 2021, 10(5): 793. doi: 10.3390/antiox10050793
|
| [27] |
Kelly PJ, Camps-Renom P, Giannotti N, et al. Carotid plaque inflammation imaged by 18F-fluorodeoxyglucose positron emission tomography and risk of early recurrent stroke[J]. Stroke, 2019, 50 (7): 1766-73. doi: 10.1161/STROKEAHA.119.025422
|
| [28] |
Mushari NA, Soultanidis G, Duff L, et al. Exploring the utility of radiomic feature extraction to improve the diagnostic accuracy of cardiac sarcoidosis using FDG PET[J]. Front Med (Lausanne), 2022, 9: 840261.
|
| [29] |
Mather KJ, DeGrado TR. Imaging of myocardial fatty acid oxidation[J]. Biochim Biophys Acta, 2016, 1861(10): 1535-43. doi: 10.1016/j.bbalip.2016.02.019
|
| [30] |
Sithara T, Drosatos K. Metabolic complications in cardiac aging[J]. Front Physiol, 2021, 12: 669497. doi: 10.3389/fphys.2021.669497
|
| [31] |
Bugge Asperhrim B. Effects of increased aortic blood pressure on myocardial performance and metabolism during non & hyphen; adrenergic inotropic stimulation of the heart[J]. Surv Anesthesiol, 1974, 18(1): 4. doi: 10.1097/00132586-197402000-00003
|
| [32] |
Dridi H, Kushnir A, Zalk R, et al. Intracellular calcium leak in heart failure and atrial fibrillation: a unifying mechanism and therapeutic target[J]. Nat Rev Cardiol, 2020, 17(11): 732-47. doi: 10.1038/s41569-020-0394-8
|
| [33] |
Karwi QG, Sun QY, Lopaschuk GD. The contribution of cardiac fatty acid oxidation to diabetic cardiomyopathy severity[J]. Cells, 2021, 10(11): 3259. doi: 10.3390/cells10113259
|
| [34] |
Kahles H, Hellige G, Hunneman DH, et al. Influence of myocardi-al substrate utilization on the oxygen consumption of the heart[J]. Clin Cardiol, 1982, 5(4): 286-93. doi: 10.1002/clc.4960050404
|
| [35] |
Yamada Y, Nakano S, Gatate Y, et al. Feasibility of simultaneous 99mTc-tetrofosmin and 123I-BMIPP dual-tracer imaging with cadmium-zinc-telluride detectors in patients undergoing primary coronary intervention for acute myocardial infarction[J]. J Nucl Cardiol, 2021, 28(1): 187-95. doi: 10.1007/s12350-018-01585-9
|
| [36] |
Cai Z, Mason NS, Anderson CJ, et al. Synthesis and preliminary evaluation of an 18F-labeled oleic acid analog for PET imaging of fatty acid uptake and metabolism[J]. Nucl Med Biol, 2016, 43(1): 108-15. doi: 10.1016/j.nucmedbio.2015.08.005
|
| [37] |
Zhao H, Shui B, Zhao Q, et al. Quantitative metabolomics reveals heart failure with midrange ejection fraction as a distinct phenotype of heart failure[J]. Can J Cardiol, 2021, 37(2): 300-9. doi: 10.1016/j.cjca.2020.03.024
|
| [38] |
Huber JS, Hernandez AM, Janabi M, et al. Longitudinal evaluation of myocardial fatty acid and glucose metabolism in fasted and nonfasted spontaneously hypertensive rats using MicroPET/CT[J]. Mol Imaging, 2017, 16: 724558.
|
| [39] |
Bergmann SR. Imaging of myocardial fatty acid metabolism with PET[J]. J Nucl Cardiol, 2007, 14(3): S118-24. doi: 10.1016/j.nuclcard.2007.02.007
|
| [40] |
de Jong HWAM, Rijzewijk LJ, Lubberink M, et al. Kinetic models for analysing myocardial[11C]palmitate data[J]. Eur J Nucl Med Mol Imaging, 2009, 36(6): 966-78. doi: 10.1007/s00259-008-1035-3
|
| [41] |
Lauritsen KM, Nielsen BRR, Tolbod LP, et al. SGLT2 inhibition does not affect myocardial fatty acid oxidation or uptake, but reduces myocardial glucose uptake and blood flow in individuals with type 2 diabetes: a randomized double-blind, placebo-controlled crossover trial[J]. Diabetes, 2021, 70(3): 800-8. doi: 10.2337/db20-0921
|
| [42] |
Chiu LS, Pedley A, Massaro JM, et al. The association of non-alcoholic fatty liver disease and cardiac structure and function-Framingham Heart Study[J]. Liver Int, 2020, 40(10): 2445-54. doi: 10.1111/liv.14600
|
| [43] |
Dewey M, Siebes M, Kachelrieß M, et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia[J]. Nat Rev Cardiol, 2020, 17(7): 427-50. doi: 10.1038/s41569-020-0341-8
|
| [44] |
Topping GJ, Yung A, Schaffer P, et al. Manganese concentration mapping in the rat brain with MRI, PET, and autoradiography[J]. Med Phys, 2017, 44(8): 4056-67. doi: 10.1002/mp.12300
|
| [45] |
Dubash S, Keat N, Kozlowski K, et al. Clinical translation of 18F-fluoropivalate-a PET tracer for imaging short-chain fatty acid metabolism: safety, biodistribution, and dosimetry in fed and fasted healthy volunteers[J]. Eur J Nucl Med Mol Imaging, 2020, 47(11): 2549-61. doi: 10.1007/s00259-020-04724-y
|
| [46] |
Piccinelli M, Cooke DC, Garcia EV. Multimodality image fusion for coronary artery disease detection: concepts and latest developments[J]. Ann Nucl Cardiol, 2018, 4(1): 74-8. doi: 10.17996/anc.18-00065
|
| [47] |
Tu ZD, Li SH, Sharp TL, et al. Synthesis and evaluation of 15-(4-(2-[18F]fluoroethoxy)phenyl)pentadecanoic acid: a potential PET tracer for studying myocardial fatty acid metabolism[J]. Bioconjugate Chem, 2010, 21(12): 2313-9. doi: 10.1021/bc100343h
|
| [48] |
Rebelos E, Hirvonen J, Bucci M, et al. Brain free fatty acid uptake is elevated in morbid obesity, and is irreversible 6 months after bariatric surgery: a positron emission tomography study[J]. Diabetes Obes Metab, 2020, 22(7): 1074-82. doi: 10.1111/dom.13996
|
| [49] |
Takala T, Nuutila P, Pulkki K, et al. 14(R, S)-[18F]Fluoro-6-thia-heptadecanoic acid as a tracer of free fatty acid uptake and oxidation in myocardium and skeletal muscle[J]. Eur J Nucl Med, 2002, 29(12): 1617-22. doi: 10.1007/s00259-002-0979-y
|
| [50] |
Sekiguchi K, Kanazu T, Murayama N, et al. In vitro inhibition and enhancement of liver microsomal S-777469 metabolism by long-chain fatty acids and serum albumin: insight into in vitro and in vivo discrepancy of metabolite formation in humans[J]. Xenobiotica, 2016, 46(6): 495-502. doi: 10.3109/00498254.2015.1091114
|
| [51] |
Taylor M, Wallhaus TR, Degrado TR, et al. An Evaluation of Myocardial Fatty Acid and Glucose Uptake Using PET with 18F-Fluoro-6-Thia-Heptadecanoic Acid and 18F-FDG in Patients with Congestive Heart Failure[J]. J Nuclear Med, 2001, 42(1): 55-62.
|
| [52] |
Zhao L, Pang Y, Luo Z, et al. Role of 68Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with[18F]-FDG PET/CT[J]. Eur J Nuclear Med Molecular Imaging, 2021, 48(6): 1944-55. doi: 10.1007/s00259-020-05146-6
|
| [53] |
Effendi N, Mishiro K, Wakabayashi H, et al. Synthesis and evaluation of radiogallium-labeled long-chain fatty acid derivatives as myocardial metabolic imaging agents[J]. PLoS One, 2021, 16 (12): e0261226. doi: 10.1371/journal.pone.0261226
|
| [54] |
Jain A, Mathur A, Pandey U, et al. 68Ga labeled fatty acids for cardiac metabolic imaging: influence of different bifunctional chelators[J]. Bioorg Med Chem Lett, 2016, 26(23): 5785-91. doi: 10.1016/j.bmcl.2016.10.048
|






 下载:
下载: