Curative effects of whole brain radiotherapy with or without simultaneous integrated boost for more than 3 brain metastases
-
摘要:
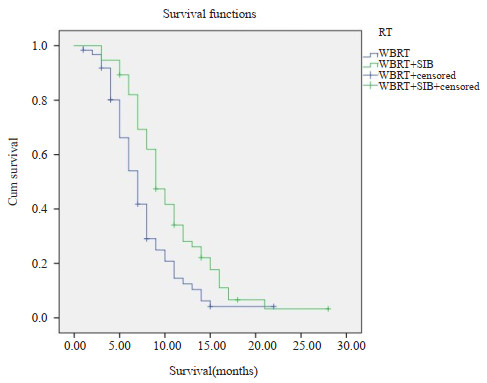
目的 对比全脑放疗(WBRT)与全脑加局部同期推量放疗(WBRT+SIB)在大于3个脑转移病灶患者中的治疗效果及毒副作用。 方法 对2011年7月~2020年7月就诊于深圳市人民医院肿瘤放疗科的118例脑转移瘤患者进行回顾性分析,根据放疗方法的不同将患者分为WBRT组(n=62)和WBRT+SIB组(n=56)。分别观察两组的生存率和毒副反应。 结果 WBRT+SIB组与WBRT组的中位生存时间分别为10.7月和7.8月,1年生存率分别为32%和13%(P=0.02);RPA 1分者WBRT+SIB和WBRT中位生存时间分别为13.6月和10.5月,1年生存率分别为51%和28%(P=0.01);RPA 2分者WBRT+SIB和WBRT中位生存时间分别为9.3月和6.8月,1年生存率分别为18%和11%(P=0.39)。两组各项早期及晚期毒副反应的差异无统计学意义(P>0.05)。 结论 对大于3个脑转移瘤的患者,WBRT+SIB生存率优于WBRT,副作用无差异。 Abstract:Objective To compare the therapy effect and toxic side effects of whole brain radiotherapy and whole brain with or without simultaneous integrated boost in patients with more than 3 brain metastatic lesions. Methods Patients with more than 3 brain metastatic lesions undergoing radiotherapy in the Department of Radiation Oncology of Shenzhen People's Hospital from July 2011 to July 2020 were analyzed retrospectively, which grouped to whole brain radiotherapy (WBRT group, n=62) and whole brain radiotherapy plus simultaneous integrated boost radiotherapy (WBRT + SIB group, n=56). We observed the survival rate and toxic side effects of the two groups. Results The median survival time of WBRT+SIB group and the WBRT group were 10.7 months and 7.8 months, respectively. The 1-year survival rate were 32% and 13% (P=0.02), respectively. The median survival time of WBRT+SIB and WBRT for those with RPA 1 point were 13.6 months and 10.5 months, and the 1-year survival rates were 51% and 28% (P=0.01). The median survival time of WBRT + SIB and WBRT for those with RPA 2 points were 9.3 months and 6.8 months, and the 1- year survival rates were 18% and 11% (P=0.39). There were no significant differences of the early and late toxic side reactions between the two groups(P>0.05). Conclusion For patients with more than 3 brain metastases, the survival rate of WBRT+SIB was better than whole brain radiotherapy, and there were no difference in side effects. -
表 1 患者临床特征
Table 1. Clinical characteristics of patients (n)
Characteristics WBRT(n=62) WBRT+SIB(n=56) P Age (years) 0.33 ≥60 38 26 <60 24 30 Gender 0.75 Male 29 24 Female 33 32 Primary disease 0.09 Lung cancer 37 32 Breast cancer 16 14 Melanoma 1 3 Renal cancer 2 2 Colon cancer 3 2 Ovarian cancer 1 2 Gastric cancer 2 1 Karnofsky score 0.52 90-100 35 31 70-80 27 25 RPA score 0.17 1 18 17 2 44 39 Metastasis 0.35 Only brain 15 11 Brain with extracrabial 47 45 Tumor diameter (cm) 0.19 ≥3 12 10 <3 50 46 WBRT: Whole brain radiotherapy; SIB: Simultaneous integrated boost. 表 2 急性毒副反应
Table 2. early reaction (n)
Early reaction WBRT WBRT+SIB P Grade Ⅰ Grade Ⅱ Grade Ⅲ Grade Ⅳ Grade Ⅰ Grade Ⅱ Grade Ⅲ Grade Ⅳ Nausea and vomiting 32 7 0 0 27 5 1 0 0.63 Skin 59 3 0 0 52 4 0 0 0.13 Headache and dizziness 43 4 0 0 35 5 2 0 0.72 Ataxia 0 1 0 0 1 0 0 0 0.32 Muscle weakness 5 2 0 0 3 3 1 0 0.91 Drowsiness 0 0 1 0 0 1 0 0 0.37 表 3 晚期毒副反应
Table 3. Late reaction (n)
Late reaction WBRT WBRT+SIB P Grade Ⅰ Grade Ⅱ Grade Ⅲ Grade Ⅳ Grade Ⅰ Grade Ⅱ Grade Ⅲ Grade Ⅳ Nausea and vomiting 1 0 0 0 2 1 0 0 0.31 Skin 1 0 0 0 2 0 0 0 0.35 Headache and dizziness 11 4 0 0 13 3 1 0 0.87 Ataxia 0 1 0 0 1 0 0 0 0.18 Muscle weakness 3 1 0 0 2 1 0 0 0.07 Drowsiness 1 0 0 0 0 0 0 0 0.24 表 4 两种放疗前后肿瘤标志物下降幅度
Table 4. Decrease of tumor markers before and after two kinds of radiotherapy (%, Mean±SD)
Tumor markers WBRT WBRT+SIB t P CEA(μg/L) 6±8 5±6 2.67 0.62 CA125(U/mL) 8±6 9±8 3.22 0.87 CA199(U/mL) 11±14 7±12 2.87 0.46 -
[1] Pruitt AA. Epidemiology, treatment, and complications of central nervous system metastases[J]. Continuum (Minneap Minn), 2017, 23(6): 1580-600. [2] Soffietti R, Rudā R, Mutani R. Management of brain metastases[J]. J Neurol, 2002, 249(10): 1357-69. doi: 10.1007/s00415-002-0870-6 [3] Weissman DE. Glucocorticoid treatment for brain metastases and epidural spinal cord compression: a review[J]. J Clin Oncol, 1988, 6(3): 543-51. doi: 10.1200/JCO.1988.6.3.543 [4] Diener-West M, Dobbins TW, Phillips TL, et al. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using rtog study 7916[J]. Int J Radiat Oncol, 1989, 16(3): 669-73. doi: 10.1016/0360-3016(89)90483-5 [5] Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the radiation therapy oncology group[J]. Int J Radiat Oncol, 1980, 6(1): 1-9. doi: 10.1016/0360-3016(80)90195-9 [6] Wen PY, Loeffler JS. Brain metastases[J]. Curr Treat Options in On-col, 2000, 1(5): 447-57. doi: 10.1007/s11864-000-0072-3 [7] Graber JJ, Cobbs CS, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines on the use of ste-reotactic radiosurgery in the treatment of adults with metastatic brain tumors[J]. Neurosurgery, 2019, 84(3): E168-70. doi: 10.1093/neuros/nyy543 [8] Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cogni-tive function in patients with 1 to 3 brain metastases: a randomized clinical trial[J]. JAMA, 2016, 316(4): 401-9. doi: 10.1001/jama.2016.9839 [9] Lippitz B, Lindquist C, Paddick I, et al. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence[J]. Cancer Treat Rev, 2014, 40(1): 48-59. doi: 10.1016/j.ctrv.2013.05.002 [10] Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prog-nostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4, 259 patients[J]. Int J Radiat Oncol, 2010, 77(3): 655-61. doi: 10.1016/j.ijrobp.2009.08.025 [11] Brown PD, Gondi V, Pugh S, et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: Phase Ⅲ trial NRG Oncology CC001[J]. J Clin Oncol, 2020, 38(10): 1019-29. doi: 10.1200/JCO.19.02767 [12] Stelzer K. Epidemiology and prognosis of brain metastases[J]. Surg Neurol Int, 2013, 4(5): 192. doi: 10.4103/2152-7806.111296 [13] Lehrer EJ, Peterson JL, Zaorsky NG, et al. Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta- analysis of 24 trials[J]. Int J Radiat Oncol, 2019, 103(3): 618-30. doi: 10.1016/j.ijrobp.2018.10.038 [14] Minniti G, Scaringi C, Lanzetta G, et al. Comparative effectiveness of multi-fraction stereotactic radiosurgery for surgically resected or intact large brain metastases from non-small-cell lung cancer (NSCLC)[J]. Lung Cancer, 2019, 132: 119-25. doi: 10.1016/j.lungcan.2019.04.021 [15] McTyre E, Ayala-Peacock D, Contessa J, et al. Multi-institutional competing risks analysis of distant brain failure and salvage pat-terns after upfront radiosurgery without whole brain radiotherapy for brain metastasis[J]. Ann Oncol, 2018, 29(2): 497-503. doi: 10.1093/annonc/mdx740 [16] David WA, Charles BS, Paul WS, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase Ⅲ results of the RTOG 9508 randomised trial[J]. Lancet, 2004, 363(9422): 1665-72. doi: 10.1016/S0140-6736(04)16250-8 [17] Dobi , Fodor E, Maráz A, et al. Boost irradiation integrated to whole brain radiotherapy in the management of brain metastases [J]. Pathol Oncol Res, 2020, 26(1): 149-57. doi: 10.1007/s12253-018-0383-y [18] Gaspar LE, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases[J]. Int J Radiat Oncol, 2000, 47(4): 1001-6. doi: 10.1016/S0360-3016(00)00547-2 [19] Miller JA, Kotecha R, Ahluwalia MS, et al. Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies[J]. Cancer, 2017, 123(12): 2283-93. doi: 10.1002/cncr.30616 [20] Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metas-tases: an analysis of 1, 960 patients in the RTOG database[J]. Int J Radiat Oncol, 2008, 70(2): 510-4. doi: 10.1016/j.ijrobp.2007.06.074 [21] Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases[J]. JAMA Oncol, 2017, 3(6): 827. doi: 10.1001/jamaoncol.2016.3834 [22] Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study[J]. Lancet Oncol, 2014, 15(4): 387-95. doi: 10.1016/S1470-2045(14)70061-0 [23] Hughes RT, Masters AH, McTyre E, et al. Initial SRS for patients with 5-15 brain metastases: results of a multi-institutional experi-ence[J]. Int J Radiat Oncol, 2018, 102(3): S141-2. [24] Patel KR, Burri SH, Boselli D, et al. Comparing pre- operative stereotactic radiosurgery (SRS) to post- operative whole brain radiation therapy (WBRT) for resectable brain metastases: a multi-institutional analysis[J]. J Neurooncol, 2017, 131(3): 611-8. doi: 10.1007/s11060-016-2334-3 [25] Routman DM, Yan E, Vora S, et al. Preoperative stereotactic radio-surgery for brain metastases[J]. Front Neurol, 2018, 9: 959. doi: 10.3389/fneur.2018.00959 [26] Lnts with limited brain metastases treated with hypofractionated (5 × 6 Gy) conformal radiotherapy[J]. Radiother Oncol, 2017, 123(2): 203-8. [27] Hotta K, Segawa Y, Takigawa N, et al. Evaluation of the relation-ship between serum carcinoembryonic antigen level and treatment outcome in surgically resected clinical-stage Ⅰ patients with non-small-cell lung cancer[J]. Anticancer Res, 2000, 20(2B): 2177-80. [28] Arrieta O, Saavedra-Perez D, Kuri R, et al. Brain metastasis development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell lung cancer: a prospective analysis[J]. BMC Cancer, 2009, 9(1): 1-9. doi: 10.1186/1471-2407-9-1 [29] Huang Z, Xu D, Zhang F, et al. Pro- gastrin-releasing peptide and neuron-specific enolase: useful predictors of response to chemotherapy and survival in patients with small cell lung cancer [J]. Clin Transl Oncol, 2016, 18(10): 1019-25. doi: 10.1007/s12094-015-1479-4 [30] Shao YB, Sun XF, He YN, et al. Elevated levels of serum tumor markers CEA and CA15- 3 are prognostic parameters for different molecular subtypes of breast cancer[J]. PLoS One, 2015, 10(7): e0133830. doi: 10.1371/journal.pone.0133830 [31] Heo MH, Cho YJ, Kim HK, et al. Isolated pachymeningeal metastasis from breast cancer: clinical features and prognostic factors[J]. Breast, 2017, 35: 109-14. doi: 10.1016/j.breast.2017.07.006 [32] Lee DS, Kim YS, Jung SL, et al. The relevance of serum carcinoembryonic antigen as an indicator of brain metastasis detection in advanced non- small cell lung cancer[J]. Tumor Biol, 2012, 33(4): 1065-73. doi: 10.1007/s13277-012-0344-0 [33] Bidard FC, Hajage D, Bachelot T, et al. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study[J]. Breast Cancer Res, 2012, 14(1): 1-10. doi: 10.1186/bcr3082 [34] Wang X, Ma KW, Yang ZG, et al. Systematic correlation analyses of circulating tumor cells with clinical variables and tumor markers in lung cancer patients[J]. J Cancer, 2017, 8(15): 3099-104. doi: 10.7150/jca.18032 [35] Ishibashi N, Maebayashi T, Aizawa T, et al. Serum tumor marker levels at the development of intracranial metastasis in patients with lung or breast cancer[J]. J Thorac Dis, 2019, 11(5): 1765-71. doi: 10.21037/jtd.2019.05.37 [36] Ushio Y, Shimizu K, Aragaki Y, et al. Alteration of blood-CSF barrier by tumor invasion into the meninges[J]. J Neurosurg, 1981, 55(3): 445-9. doi: 10.3171/jns.1981.55.3.0445 [37] Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: review and update on management[J]. Cancer, 2018, 124(1): 21-35. doi: 10.1002/cncr.30911 [38] Yuan H, Gaber MW, Boyd K, et al. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes[J]. Int J Radiat Oncol, 2006, 66(3): 860-6. doi: 10.1016/j.ijrobp.2006.06.043 [39] Yoshida Y, Sejimo Y, Kurachi M, et al. X-ray irradiation induces disruption of the blood-brain barrier with localized changes in claudin- 5 and activation of microglia in the mouse brain[J]. Neurochem Int, 2018, 119: 199-206. doi: 10.1016/j.neuint.2018.03.002 [40] Murrell DH, Zarghami N, Jensen MD, et al. Evaluating changes to blood-brain barrier integrity in brain metastasis over time and after radiation treatment[J]. Transl Oncol, 2016, 9(3): 219-27. doi: 10.1016/j.tranon.2016.04.006 -







 下载:
下载: