Factors affecting the diagnostic performance of suspicious amorphous calcification
-
摘要:
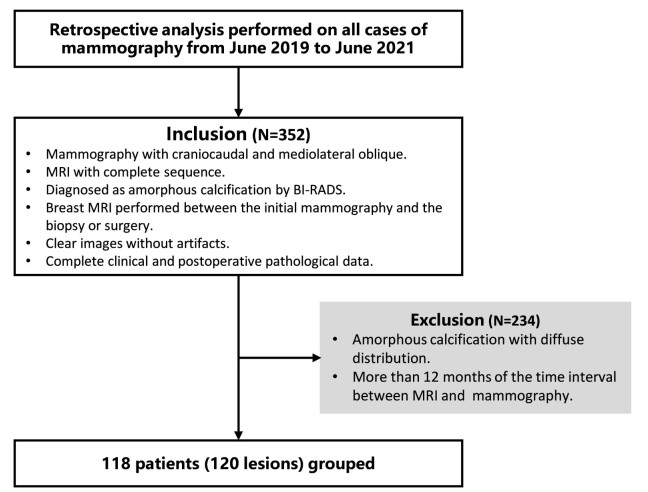
目的 探讨影响可疑无定形钙化诊断正确性的因素,并通过定量分析评估各影响因素的诊断性能。 方法 回顾性研究2019年6月1日~2021年6月1日进行乳腺X线检查的118例患者(120个病灶),患者均为女性,年龄46.7±9.7岁,中位年龄46岁。由2位工作10年以上从事乳腺影像诊断的医师对所有病例进行双盲诊断,入组病例依照2013年第5版乳腺影像报告和数据系统(BI-RADS)作为诊断标准进行描述分类,以病理结果为金标准,通过诊断试验评价指标及ROC曲线分析确定各种影响因素对可疑无定形钙化良恶性结果判断的诊断性能。 结果 乳腺动态增强磁共振(DCE-MRI)对可疑无定形钙化敏感性、特异性、阳性预测值和阴性预测值分别为100%、71.4%、71.4%、100%,曲线下面积为0.857(95% CI:0.782~0.914)。DCE-MRI检出了所有的恶性病变,点状强化病例在DCE-MRI显示的3种强化类型中诊断特异性最高,为94.4%,曲线下面积最大,为0.972(95% CI:0.785~1.000,P < 0.05)。在全体入组病例中,可疑无定形钙化恶性率为41.7%;而在无伴随征象的纯钙化组中,可疑无定形钙化的恶性率则降为24%。在钙化分布类型中,集群及区域分布的可疑无定形钙化恶性率在BI-RADS 4B等级,曲线下面积分别为0.895(95% CI:0.798~0.955)、0.815(95%CI:0.650~0.924),区域分布病例的恶性率最低为25%,在其中的纯钙化组中,恶性率则降至8%。 结论 乳腺DCE-MRI具有高敏感度,检测出所有的恶性病灶,其中点状强化病灶诊断特异性最高。在影响可疑无定形钙化诊断的诸多因素中,纯钙化组比有伴随征象组诊断特异性明显提高,对可疑无定形钙化良恶性的判断有很大的价值。钙化分布中,区域分布的纯钙化组恶性率最低,可以选择定期临床随访,从而避免一些不必要的活检或手术。 Abstract:Objective To explore the factors that affect the accuracy of diagnosis of suspicious amorphous calcification and explore while the diagnostic performance of each factor is quantitatively evaluated. Methods A retrospective study was conducted on a selected group of 118 patients (120 lesions), who underwent mammography from June 1, 2019 to June 1, 2021. All patients were female, with an average age of 46.7±9.7 years old and a median age of 46 years old. A double-blind diagnosis of all cases was performed by two doctors who engaged in breast imaging diagnosis for more than 10 years. The patients were described and classified according to the 5th edition of breast imaging reporting and diagnostic system (BI-RADS) in 2013 as diagnostic criteria, while the pathological findings were chosen as the gold standard. The evaluation indexes of diagnostic tests and ROC curves were used to analyze and determine the diagnostic performance of each affecting factor on the benign and malignant results of suspicious amorphous calcification. Results The sensitivity, the specificity, the positive predictive value and the negative predictive value of breast dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) for suspicious amorphous calcification were 100%, 71.4%, 71.4% and 100% respectively. The area under curve was 0.857 (95%CI: 0.782-0.914). All the malignant lesions were correctly diagnosed. The diagnostic specificity of the focus and foci enhancement cases was 94.4% and the area under curve was 0.972 (95% CI: 0.785-1.000, P < 0.05), that were both largest among the three types of enhancement demonstrated by DCE-MRI. The malignancy rate of suspicious amorphous calcification was 41.7% in all patients, but decreased to 24% in the pure calcification group without associated features. When considering the four kinds of calcification distribution types, only the malignancy rate of suspicious amorphous calcification with grouped and regional distribution was in BI- RADS 4B, and the area under curve of them were 0.895 (95% CI: 0.798- 0.955) and 0.815 (95% CI: 0.650-0.924), respectively. The malignancy rate of the cases with regional distribution was the lowest as 25% among all distribution types. It could even drop to 8% in the pure calcification group of it. Conclusion Breast DCE- MRI proves sensitive enough to diagnose all malignant lesions correctly and has the highest diagnostic specificity for the focus and foci enhancement cases. Among the factors affecting the diagnosis of suspicious amorphous calcification, the diagnosis specificity of pure calcification group is significantly higher than that of the cases with associated features, which is of great value for judging the benign and malignant of suspicious amorphous calcification. The cases with pure calcification in regional distribution group have lowest rate of malignancy. The regular clinical follow-up can be chosen to avoid some unnecessary biopsy or surgery. -
图 3 经乳腺DCE-MRI判断为恶性可疑无定形钙化病例
Figure 3. The case which was diagnosed malignant suspicious amorphous calcification by DCE- MRI. A: Craniocaudal; B: Mediolateral oblique and local magnification of suspected amorphous calcification (3×magnification); C: Axial T1WI sequence; D: Axial TIRM sequence; E: Axial DWI sequence; F: Axial DCE sequence; G: Sagittal DCE sequence.
图 4 经乳腺DCE-MRI判断为良性可疑无定形钙化病例
Figure 4. The case which was diagnosed benign suspicious amorphous calcification by DCE-MRI. A: Craniocaudal and local magnification of suspected amorphous calcification (3×magnification); B: Mediolateral oblique; C: Axial T1WI sequence; D: Axial TIRM sequence; E: Axial DWI sequence; F: Axial DCE sequence; G: Sagittal DCE sequence.
表 1 患者一般资料
Table 1. General data of patients [n(%)]
General data Total (n=118) Benign (n=68) Malignant (n=50) P Age (years, Mean±SD) 46.7±9.7 45.2±8.3 48.7±10.9 0.060 Menopausal status 0.017 Premenopausal 89(75.4) 57(83.8) 32(64.0) Postmenopausal 29(24.6) 11(16.2) 18(36.0) Family history of breast cancer 0.464 Yes 8(6.8) 6(8.8) 2(4.0) No 110(93.2) 62(91.2) 48(96.0) Personal history of breast cancer 0.073 Yes 3(2.5) 0 3(6.0) No 115(97.5) 68(100.0) 47(94) 表 2 120例病灶BI-RADS分类和病理对照
Table 2. Classification and pathologic comparison of BI-RADS in 120 cases [n(%)]
Pathology Clinical management BI-RADS 1(n=29) BIRADS 2/3(n=21) BI-RADS 4(n=66) BI-RADS 5(n=4) Benign Biopsy 24(82.8) 21(100.0) 19(28.8) 0 Follow-up 5(17.2) 0 1(1.5) 0 Malignant Biopsy 0 0 46(69.7) 4(100.0) Follow-up 0 0 0 0 表 3 定量分析可疑无定形钙化良恶性判断的影响因素
Table 3. Quantitative evaluation of the benign and malignant factors of suspicious amorphous calcification.
Classification Total(n) Malignant(n) PPV (%) AUC 95% CI P Calcification distribution Grouped 70 32 45.7 0.895 0.798-0.955 < 0.001 Regional 36 9 25.0 0.815 0.650-0.924 < 0.001 Linear 3 3 100.0 - - - Segmental 11 6 54.6 0.800 0.463-0.971 0.014 Associated features No 75 18 24.0 0.904 0.813-0.960 < 0.001 Yes 45 32 71.1 0.654 0.497-0.789 0.021 DCE-MRI enhancement No 30 0 0 - - - Focus and foci 20 2 10.0 0.972 0.785-1.000 < 0.001 Masses 38 29 76.3 0.611 0.440-0.765 0.131 NME 32 19 59.4 0.538 0.354-0.715 0.317 NME: Nonmass-like enhancement; PPV: Positive predictive value. 表 4 有无伴随征象对区域及集群分布可疑无定形钙化DCE-MRI诊断性能的影响
Table 4. The influence of associated features on the diagnostic performance of DCE- MRI in suspicious amorphous calcification with regional and grouped distribution
Distribution Lesion classification Pathology PPV (%) Sensibility(%) Specificity(%) Detail Regional Total (n=36) Benign (n=27) 25 100 63 15FC; 1FT; 8OB; 3 follow-up Malignant (n=9) 1IDC; 1OM 1DCIS; 6IDC with DCIS Calcifications without associated features (n=23) Benign (n=21) 8 100 76.1 13FC; 1FT; 4OB; 3 follow-up Malignant (n=2) 1DCIS; 1OM Grouped Total (n=70) Benign (n=38) 45 100 79 28FC; 3FT; 4OB; 3 follow-up Malignant (n=32) 3IDC; 2OM 12DCIS; 15IDC with DCIS; Calcifications without associated features (n=43) Benign (n=31) 27.9 100 87.1 23FC; 1FT; 4OB; 3 follow-up Malignant (n=12) 10DCIS; 1OM; 1IDC with DCIS FC: Fibrocystic change; FT: Fibroepithelial tumors; OB: Other benign tumors; IDC: Invasive ductal carcinoma; DCIS: Ductal carcinoma in situ; OM: Other malignant tumors. -
[1] Mercado CL. BI-RADS update[J]. Radiol Clin North Am, 2014, 52 (3): 481-7. doi: 10.1016/j.rcl.2014.02.008 [2] Vinnicombe S. How I report breast magnetic resonance imaging studies for breast cancer staging and screening[J]. Cancer Imaging, 2016, 16: 17. doi: 10.1186/s40644-016-0078-0 [3] Iwase M, Tsunoda H, Nakayama K, et al. Overcalling low-risk find-ings: grouped amorphous calcifications found at screening mam-mography associated with minimal cancer risk[J]. Breast Cancer, 2017, 24(4): 579-84. doi: 10.1007/s12282-016-0742-z [4] Berg WA, Arnoldus CL, Teferra E, et al. Biopsy of amorphous breast calcifications: pathologic outcome and yield at stereotactic bi-opsy[J]. Radiology, 2001, 221(2): 495-503. doi: 10.1148/radiol.2212010164 [5] Uematsu T, Yuen S, Kasami M, et al. Dynamic contrast-enhanced MR imaging in screening detected microcalcification lesions of the breast: is there any value?[J]. Breast Cancer Res Treat, 2007, 103(3): 269-81. doi: 10.1007/s10549-006-9373-y [6] 阳宁静, 周小灵, 曹英, 等. 钼靶钙化BI-RADS 3~5类乳腺病变阳性预测值分析[J]. 成都医学院学报, 2015, 10(6): 721-4. doi: 10.3969/j.issn.1674-2257.2015.06.022 [7] Moy L. Should we continue to biopsy all amorphous calcifications? [J]. Radiology, 2018, 288(3): 680-1. doi: 10.1148/radiol.2018180767 [8] Shen L, Ma X, Jiang T, et al. Malignancy Risk Stratification Prediction of Amorphous Calcifications Based on Clinical and Mammographic Features[J]. Cancer Manag Res, 2021, 13: 235-45. doi: 10.2147/CMAR.S286269 [9] Jiang YN, Lou JJ, Wang SQ, et al. Evaluation of the role of dynamic contrast-enhanced MR imaging for patients with BI-RADS 3- 4 microcalcifications[J]. PLoS One, 2014, 9(6): e99669. doi: 10.1371/journal.pone.0099669 [10] Linda AN, Zuiani C, Londero V, et al. Role of magnetic resonance imaging in probably benign (BI-RADS category 3) microcalcifica-tions of the breast[J]. Radiol med, 2014, 119(6): 393-9. doi: 10.1007/s11547-013-0361-0 [11] Zhang M, Horvat JV, Bernard-Davila B, et al. Multiparametric MRI model with dynamic contrast-enhanced and diffusion-weighted im-aging enables breast cancer diagnosis with high accuracy[J]. J Magn Reson Imaging, 2019, 49(3): 864-74. doi: 10.1002/jmri.26285 [12] Shimauchi A, Machida Y, Maeda I, et al. Breast MRI as a problem-solving study in the evaluation of BI-RADS categories 3 and 4 microcalcifications: is it worth performing?[J]. Acad Radiol, 2018, 25(3): 288-96. doi: 10.1016/j.acra.2017.10.003 [13] Baltzer PAT, Bennani-Baiti B, Stttinger A, et al. Is breast MRI a helpful additional diagnostic test in suspicious mammographic microcalcifications?[J]. Magn Reson Imaging, 2018, 46: 70-4. doi: 10.1016/j.mri.2017.10.012 [14] 尤超, 顾雅佳, 彭卫军, 等. MRI鉴别乳腺导管原位癌与其他导管内病变的价值[J]. 中国癌症杂志, 2014, 24(6): 463-8. doi: 10.3969/j.issn.1007-3969.2014.06.011 [15] Oligane HC, Berg WA, Bandos AI, et al. Grouped amorphous calci-fications at mammography: frequently atypical but rarely associat-ed with aggressive malignancy[J]. Radiology, 2018, 288(3): 671-9. doi: 10.1148/radiol.2018172406 [16] Park GE, Kim SH, Lee JM, et al. Comparison of positive predictive values of categorization of suspicious calcifications using the 4th and 5th editions of BI-RADS[J]. AJR Am J Roentgenol, 2019, 213(3): 710-5. doi: 10.2214/AJR.18.20866 [17] Choi WJ, Han K, Shin HJ, et al. Calcifications with suspicious morphology at mammography: should they all be considered with the same clinical significance?[J]. Eur Radiol, 2021, 31(4): 2529-38. doi: 10.1007/s00330-020-07215-8 [18] Taskin F, Kalayci CB, Tuncbilek N, et al. The value of MRI contrast enhancement in biopsy decision of suspicious mammographic microcalcifications: a prospective multicenter study [J]. Eur Radiol, 2021, 31(3): 1718-26. doi: 10.1007/s00330-020-07265-y [19] Greenwood HI, Wilmes LJ, Kelil T, et al. Role of breast MRI in the evaluation and detection of DCIS: opportunities and challenges [J]. J Magn Reson Imaging, 2020, 52(3): 697-709. doi: 10.1002/jmri.26985 [20] Sumner WE, Koniaris LG, Snell SE, et al. Results of 23, 810 cases of ductal carcinoma-in-situ[J]. Ann Surg Oncol, 2007, 14(5): 1638-43. doi: 10.1245/s10434-006-9316-1 [21] Engels K, Fox SB, Whitehouse RM, et al. Distinct angiogenic patterns are associated with high-grade in situ ductal carcinomas of the breast[J]. J Pathol, 1997, 181(2): 207-12. doi: 10.1002/(SICI)1096-9896(199702)181:2<207::AID-PATH758>3.0.CO;2-4 [22] Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions?[J]. Radiology, 1999, 211(1): 101-10. doi: 10.1148/radiology.211.1.r99ap38101 [23] Bennani-Baiti B, Baltzer PA. MR imaging for diagnosis of malig-nancy in mammographic microcalcifications: a systematic review and meta-analysis[J]. Radiology, 2017, 283(3): 692-701. doi: 10.1148/radiol.2016161106 -







 下载:
下载: