Contrast-enhance computed tomography radiomics of papillary thyroid cancer to predictive the central lymph node metastases before surgery
-
摘要:
目的 探讨临床、CT影像组学及融合模型等6个模型术前预测甲状腺乳头状癌(PTC)中央区淋巴结转移(CLNM)的价值。 方法 纳入103例PTC患者,根据术后病理结果将患者分为无CLNM组(n=50)和CLNM组(n=53),比较组间临床资料及CT征象差异,按7∶3比例随机将各组分为训练集及测试集,提取训练集CT影像组学的特征,构建临床模型、平扫期(NP)模型、动脉期(AP)模型、静脉期(AP)模型、NP+AP+VP模型及融合模型。模型的效能的评价标准为AUC、敏感度及特异性。 结果 两组的性别差异有统计学意义(P=0.002);在CT征象中,两组间病灶直径(P=0.001)及甲状腺包膜侵犯(P=0.024)的差异有统计学意义。在NP模型、AP模型、VP模型及NP+AP+VP模型这4个组学模型中NP+AP+VP模型预测PTC患者发生CLNM的效能最佳。比较临床模型、NP+AP+VP模型及融合模型的预测效能,融合模型表现最佳,在训练集和测试集中均具有最高的AUC、敏感度和特异性。 结论 融合模型在训练集和测试集中预测PTC患者发生CLNM的效能均最好,有望对术前评估PTC患者CLNM提供有效的辅助手段。 Abstract:Objective To explore the value of six models established according to clinical data, CT imaging radiomics, and combining both for predicting preoperatively central lymph node metastasis (CLNM) in patients with papillary thyroid carcinoma (PTC). Methods A total of 103 PTC patients were enrolled and divided into non-CLNM group (n=50) and CLNM group (n=53) according to pathology. The clinical data and CT signatures were compared between groups. The patients in each group were randomly divided into training set and test set according at the ratio of 7:3. CT radiomics features of PTC were selected from training set, and clinical model, non-contrast phase (NP) model, arterial phase (AP) model, venous phase (VP) model, NP + AP + VP model and the combining model were constructed, respectively. AUC, sensitivity and specificity were calculated to evaluate the effectiveness of these six models. Results In clinical data, there was significant difference in gender between the two groups (P=0.002). The lesion diameter (P=0.001) and thyroid capsule invasion (P=0.024) of two groups were significant. Among the NP model, AP model, VP model and NP + AP + VP model these four radiomics models, NP + AP + VP model had the best efficacy for predicting CLNM in PTC patients. Compared with the clinical model and NP+AP+VP model, the combining model performed best and had the highest AUC, sensitivity and specificity in both training set and test set. Conclusion All of six models, the combining model high efficacy for predicting preoperatively CLNM, which is expected to provide an effective auxiliary method for preoperative the CLNM in PTC patients. -
表 1 无CLNM组和CLNM组的临床资料比较
Table 1. Comparison of clinical data between the non-CLNM group and the CLNM group (n)
临床特征 无CLNM组(n=50) CLNM组(n=53) P 年龄(岁) 0.104 < 55 27 37 ≥55 23 16 性别 0.002 男 10 26 女 40 27 伴腺瘤 0.552 有 8 4 无 42 49 伴结节性甲状腺肿 0.093 有 20 13 无 30 40 伴桥本氏甲状腺炎 0.778 有 12 14 无 42 39 CLNM: 中央区淋巴结转移. 表 2 无CLNM组和CLNM组的CT征象比较
Table 2. Comparison of CT signatures between the non-CLNM group and the CLNM group (n)
CT征象 无CLNM组(n=50) CLNM组(n=53) P 位置 0.685 右 29 23 左 19 29 峡部 1 0 双侧叶 1 1 数目 1.000 单发 49 52 多发 1 1 直径(mm) 0.001 5~10 24 9 > 10 26 44 形态 0.616 规则 23 27 不规则 27 26 钙化 0.390 有 33 39 无 17 14 增强后边界 0.488 清晰 23 28 模糊 27 25 包膜侵犯 0.024 有 24 37 无 26 16 周围侵犯 0.111 有 5 12 无 45 41 表 3 各模型纳入的组学特征
Table 3. The included CT radiomics features of each model
模型 特征 NP模型 n_square_glrlm_RunEntropy n_wavelet-LHH_firstorder_RobustMeanAbsoluteDeviation n_wavelet-LLH_gldm_SmallDependenceEmphasis n_wavelet-HHH_glcm_SumEntropy AP模型 a_wavelet-HHL_firstorder_90Percentile a_squareroot_glszm_SmallAreaEmphasis VP模型 v_wavelet-HHL_firstorder_Range v_lbp-2D_firstorder_MeanAbsoluteDeviation v_wavelet-LHL_firstorder_Median v_wavelet-LLL_glcm_MCC v_squareroot_glrlm_LowGrayLevelRunEmphasis NP+AP+VP模型 v_wavelet-HHL_firstorder_Range v_squareroot_glrlm_LowGrayLevelRunEmphasis n_wavelet-LHH_firstorder_RobustMeanAbsoluteDev a_squareroot_glszm_SmallAreaEmphasis v_lbp-2D_firstorder_MeanAbsoluteDeviation NP: 平扫期; AP: 动脉期; VP: 静脉期. 表 4 各模型预测CLNM的效能
Table 4. The effectiveness of each model for predicting CLNM
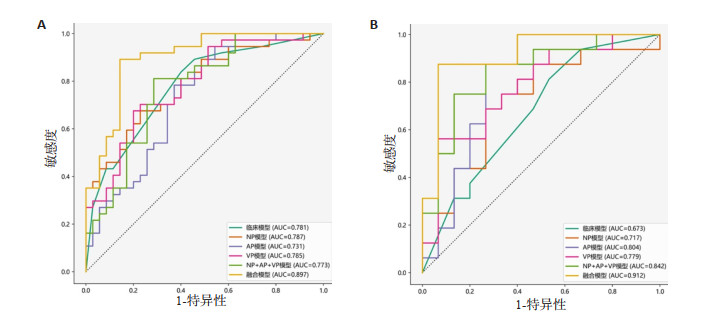
模型 训练集 测试集 AUC 95% CI 敏感度 特异性 AUC 95% CI 敏感度 特异性 临床模型 0.781 0.678~0.884 0.838 0.600 0.673 0.482~0.864 0.688 0.533 NP模型 0.787 0.683~0.891 0.676 0.771 0.717 0.527~0.906 0.875 0.500 AP模型 0.731 0.614~0.849 0.784 0.629 0.804 0.632~0.976 0.875 0.600 VP模型 0.785 0.680~0.890 0.676 0.800 0.701 0.611~0.947 0.750 0.667 NP+AP+VP模型 0.773 0.664~0.882 0.773 0.764 0.843 0.698~0.986 0.843 0.774 融合模型 0.897 0.824~0.970 0.892 0.857 0.913 0.804~1.000 0.875 0.800 -
[1] Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic? the increasing impact of overdiagnosis[J]. N Engl J Med, 2016, 375(7): 614-7. doi: 10.1056/NEJMp1604412 [2] Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020[J]. Chin Med J (Engl), 2021, 134(7): 783-91. doi: 10.1097/CM9.0000000000001474 [3] Abdullah MI, Junit SM, Ng KL, et al. Papillary thyroid cancer: genetic alterations and molecular biomarker investigations[J]. Int J Med Sci, 2019, 16(3): 450-60. doi: 10.7150/ijms.29935 [4] Huang JP, Sun W, Zhang H, et al. Use of Delphian lymph node metastasis to predict central and lateral involvement in papillary thyroid carcinoma: a systematic review and meta-analysis[J]. Clin Endocrinol (Oxf), 2019, 91(1): 170-8. [5] Md FN, et al. Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer: 13-year follow-up[J]. Ann Surg Oncol, 2019, 26(6): 1737-43. doi: 10.1245/s10434-019-07263-5 [6] Cabanillas ME, McFadden DG, Durante C. Thyroid cancer[J]. Lancet, 2016, 388(10061): 2783-95. doi: 10.1016/S0140-6736(16)30172-6 [7] 秦元, 张浩. 国内外指南中甲状腺癌颈淋巴结清扫手术范围及指征变迁[J]. 中国实用外科杂志, 2020, 40(6): 639-43. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK202006005.htm [8] de Napoli L, Matrone A, Favilla K, et al. Role of prophylactic central compartment lymph node dissection on the outcome of patients with papillary thyroid carcinoma and synchronous ipsilateral cervical lymph node metastases[J]. Endocr Pract, 2020, 26(8): 807-17. doi: 10.4158/EP-2019-0532 [9] 刘再毅, 梁长虹. 促进影像组学的转化研究[J]. 中国医学影像技术, 2017, 33(12): 1765-7. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXX201712001.htm [10] Wen XZ, Jin QM, Cen XX, et al. Clinicopathologic predictors of central lymph node metastases in clinical node-negative papillary thyroid microcarcinoma: a systematic review and meta-analysis[J]. World J Surg Oncol, 2022, 20(1): 106. doi: 10.1186/s12957-022-02573-7 [11] Song JL, Yan T, Qiu WW, et al. Clinical analysis of risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: a retrospective study of 3686 patients[J]. Cancer Manag Res, 2020, 12((2)): 2523-30. [12] 张寅, 姚永忠, 许丹丹. 基于人工智能的合并慢性淋巴细胞性甲状腺炎的甲状腺乳头状癌颈中央区淋巴结转移的早期预测系统构建[J]. 中国耳鼻咽喉头颈外科, 2021, 28(11): 664-8. https://www.cnki.com.cn/Article/CJFDTOTAL-EBYT202111002.htm [13] Feng JW, Qin AC, Ye J, et al. Predictive factors for lateral lymph node metastasis and skip metastasis in papillary thyroid carcinoma [J]. Endocr Pathol, 2020, 31(1): 67-76. doi: 10.1007/s12022-019-09599-w [14] 赵泓博, 尹昳丽, 刘畅, 等. 基于CT双期增强影像组学预测甲状腺乳头状癌淋巴结转移[J]. 放射学实践, 2021, 36(4): 458-63. https://www.cnki.com.cn/Article/CJFDTOTAL-FSXS202104013.htm [15] 何俊林, 路青, 徐昕, 等. CT影像组学特征预测甲状腺乳头状癌颈部淋巴结转移的价值研究[J]. 上海交通大学学报: 医学版, 2021, 41 (9): 1233-9. https://www.cnki.com.cn/Article/CJFDTOTAL-SHEY202109015.htm [16] 沈莎莎. 基于甲状腺癌灶CT影像组学模型预测中央组淋巴结转移的可行性研究[D]. 昆明: 昆明医科大学, 2019. [17] Li JJ, Wu XX, Mao N, et al. Computed tomography-based radiomics model to predict central cervical lymph node metastases in papillary thyroid carcinoma: a multicenter study[J]. Front Endocrinol (Lausanne), 2021, 12: 741698. [18] 高鑫宇, 刘慧楠, 裴建国. 增强CT纹理分析在老年甲状腺乳头状癌诊断和预测淋巴结转移中的价值[J]. 中国老年学杂志, 2022, 42(6): 1361-4. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLXZ202206025.htm [19] Kim SK, Chai YJ, Park I, et al. Nomogram for predicting central node metastasis in papillary thyroid carcinoma[J]. J Surg Oncol, 2017, 115(3): 266-72. [20] 刘妮, 谢元亮, 黄增发, 等. 基于CT增强影像组学模型预测甲状腺乳头状癌颈部淋巴结转移[J]. 放射学实践, 2021, 36(8): 971-5. https://www.cnki.com.cn/Article/CJFDTOTAL-FSXS202108009.htm [21] Johnstone IM, Titterington DM. Statistical challenges of highdimensional data[J]. Phil Trans R SocA, 2009, 367(1906): 4237-53. [22] Clarke R, Ressom HW, Wang AT, et al. The properties of highdimensional data spaces: implications for exploring gene and protein expression data[J]. Nat Rev Cancer, 2008, 8(1): 37-49. -







 下载:
下载: