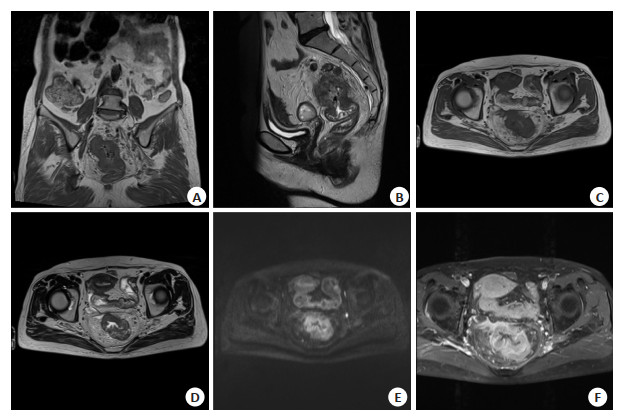
Value of the model based on MRI and microRNA-7106-3p for evaluating the efficacy of neoadjuvant chemoradiotherapy in advanced rectal cancer: a prospective study based on 127 patients
-
摘要:
目的 构建基于MRI和微小核糖核酸-7106-3p(miR-7106-3p)的列线图回归模型,探究其评价进展期直肠癌新辅助放化疗(NCRT)疗效的价值。 方法 前瞻性纳入2019年3月~2022年2月于河北省退役军人总医院就诊的直肠癌患者127例,NCRT后行全直肠系膜切除术。NCRT前1周内行MRI检查并抽取患者外周血,采用实时荧光定量聚合酶链反应法检测血清miR-7106-3p水平。进展期直肠癌NCRT的风险因素用Logistic回归分析。构建风险因素的列线图回归模型,采用决策曲线分析法、一致性指数和校准曲线评价模型进行分析。 结果 37例患者(29.13%)经NCRT后达到病理学完全缓解(pCR)(pCR组),90例(70.87%)未达到pCR(非pCR组)。pCR组的容积转运常数(Ktrans)、血管外细胞外间隙容积比(Ve)、回流速率常数(Kep)和miR-7106-3p水平均高于非pCR组,表观弥散系数(ADC)低于非pCR组(P < 0.05)。Logistic回归分析结果显示,N分期越高、ADC > 0.92×10-3 mm2/s是进展期直肠癌NCRT后pCR的独立危险因素(P < 0.05),Ktrans > 0.33 min、Ve > 0.55%、Kep > 0.54 min和miR-7106-3p > 0.31是进展期直肠癌NCRT后pCR的独立保护因素(P < 0.05)。模型C(由N分期、Ktrans、Ve、Kep、ADC和miR-7106-3p构成)的一致性指数为0.977,高于模型A(由Ktrans、Ve、Kep和ADC构成,0.957)和模型B(由N分期、Ktrans、Ve、Kep和ADC构成,0.956)。模型B的平均绝对误差为0.015,低于模型A(0.017)和模型C(0.024)。阈值概率在0.10~1.0的大部分范围内,模型C的净收益高于模型A和模型B。 结论 基于MRI和miR-7106-3p的模型能较好的评价进展期直肠癌NCRT疗效。 -
关键词:
- 进展期直肠癌 /
- 新辅助放化疗 /
- 磁共振成像 /
- 微小RNA-7106-3p /
- 列线图回归模型
Abstract:Objective To construct a nomogram regression model based on MRI and microRNA-7106-3p (miR-7106-3p), and investigate its value in evaluating the efficacy of neoadjuvant chemoradiotherapy (NCRT) for advanced rectal cancer. Methods A total of 127 patients with rectal cancer were prospectively enrolled from March 2019 to February 2022 at Hebei General Hospital For Veterans who underwent total rectal mesenteric resection after NCRT. MRI was performed within 1 week before NCRT. Peripheral blood was extracted from patients at the same time. The serum miR-7106-3p level was detected by real-time fluorescence quantitative polymerase chain reaction. Risk factors for the efficacy of NCRT in advanced rectal cancer were analysed by Logistic regression. The nomogram regression model for risk factors was constructed. Consistency index, calibration curve and decision curve analysis were used to evaluate the value of the model. Results There were 37 (29.13%) patients achieved pathological complete remission (pCR) after NCRT (pCR group), and 90 patients (70.87%) did not achieved pCR (non-pCR group). The levels of volume transport constant (Ktrans), extravascular extracellular space volume ratio (Ve), reflux rate constant (Kep) and miR-7106-3p were higher in the pCR group than in the non-pCR group, and apparent dispersion coefficient (ADC) was lower than in the non-pCR group (P < 0.05). The logistic regression analysis showed that higher N stage and ADC > 0.92×10-3 mm2/s were independent risk factors for pCR after NCRT in progressive rectal cancer (P < 0.05), and Ktrans > 0.33 min, Ve > 0.55%, Kep > 0.54 min and miR-7106-3p > 0.31 were independent protective factors for pCR after NCRT in progressive rectal cancer (P < 0.05). The consistency index of model C (consisting of N-stage, Ktrans, Ve, Kep, ADC and miR-7106-3p) was 0.977, which was higher than that of model A (consisting of Ktrans, Ve, Kep and ADC, 0.957) and model B (consisting of N-stage, Ktrans, Ve, Kep and ADC, 0.956). The mean absolute error of model B was 0.015, which was lower than that of model A (0.017) and model C (0.024). When the threshold probabilities ranged from 0.10-1.0 for most of the range, the net benefit of model C was higher than that of model A and model B. Conclusion The model can better evaluate the efficacy of NCRT in progressive rectal cancer based on MRI and miR-7106-3p. -
表 1 两组患者一般资料比较
Table 1. Comparison of clinical data between the two groups [n(%)]
项目 pCR组(n=37) 非pCR组(n=90) Z/χ2 P 年龄(岁)* 53(43,59) 54(44,58) 0.016 0.987 性别 0.132 0.716 男 23(62.16) 59(65.56) 女 14(37.84) 31(34.44) T分期 2.359 0.125 T3 29(78.38) 58(64.44) T4 8(21.62) 32(35.56) N分期 3.314 0.191 N0 26(70.27) 48(53.33) N1 8(21.62) 27(30.00) N2 3(8.11) 15(16.67) *以中位数和四分位数间距表示. 表 2 两组MRI参数和miR-7106-3p水平比较
Table 2. Comparison of MRI parameters and miR-7106-3p levels between the two groups
项目 pCR组(n=37) 非pCR组(n=90) Z/t P Ktrans(min)* 0.38(0.34,0.41) 0.33(0.31,0.34) 6.092 < 0.001 Ve(%, Mean±SD) 0.62±0.06 0.53±0.07 7.189 < 0.001 Kep(min, Mean±SD) 0.59±0.07 0.52±0.09 4.346 < 0.001 ADC(×10-3 mm2/s)* 0.89(0.86, 0.91) 0.92(0.89, 0.95) 3.710 < 0.001 miR-7106-3p* 0.34(0.32, 0.45) 0.28(0.21, 0.34) 4.837 < 0.001 *以中位数和四分位数间距表示. Ve: 血管外细胞外间隙容积比; Kep: 回流速率常数; Ktrans: 容积转运常数; ADC: 表观弥散系数. 表 3 变量赋值
Table 3. Variable assignment
变量 赋值 T分期 T3期=0,T4期=1 N分期 N0期=0,N1期=1,N2期=2 Ktrans(min) ≤0.33(中位数)=0,> 0.33=1 Ve(%) ≤0.55(中位数)=0,> 0.55=1 Kep(min) ≤0.54(中位数)=0,> 0.54=1 ADC(×10-3 mm2/s) ≤0.92(中位数)=0,> 0.92=1 miR-7106-3p ≤0.31(中位数)=0,> 0.31=1 病理学结果 pCR=0,非pCR=1 表 4 进展期直肠癌NCRT风险因素的logistic回归分析结果
Table 4. Logistic regression analysis of NCRT risk factors in advanced rectal cancer
因素 β SE Wald P OR(95% CI) T分期 1.380 0.886 2.424 0.120 3.975(0.700~22.588) N分期 1.134 0.568 3.989 0.046 3.109(1.021~9.465) Ktrans -2.914 0.853 11.672 0.001 0.054(0.010~0.289) Ve -3.131 0.904 11.980 0.001 0.044(0.007~0.257) Kep -1.976 0.829 5.682 0.017 0.139(0.027~0.704) ADC 3.549 1.085 10.695 0.001 34.770(4.145~291.674) miR-7106-3p -3.865 1.166 10.991 0.001 0.021(0.002~0.206) -
[1] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. doi: 10.3322/caac.21492 [2] Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021[J]. CA ACancer J Clin, 2021, 71(1): 7-33. doi: 10.3322/caac.21654 [3] 中华人民共和国国家卫生健康委员会. 国家卫生健康委员会中国结直肠癌诊疗规范(2020年版)[J]. 中华外科杂志, 2020, 58(8): 561-85. doi: 10.3760/cma.j.cn112139-20200518-00390 [4] Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and Meta-analysis of treatment outcomes[J]. Ann Surg, 2020, 271(3): 440-8. doi: 10.1097/SLA.0000000000003471 [5] Fernandez LM, São Julião GP, Figueiredo NL, et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: a retrospective, international, multicentre registry study[J]. Lancet Oncol, 2021, 22 (1): 43-50. doi: 10.1016/S1470-2045(20)30557-X [6] Zhou JL, Wang CX, Lin GL, et al. Serial circulating tumor DNA in predicting and monitoring the effect of neoadjuvant chemoradiotherapy in patients with rectal cancer: a prospective multicenter study[J]. Clin Cancer Res, 2021, 27(1): 301-10. doi: 10.1158/1078-0432.CCR-20-2299 [7] Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial [J]. J Clin Oncol, 2014, 32(15): 1554-62. doi: 10.1200/JCO.2013.54.3769 [8] 董健, 谢宗源, 李垣婕, 等. 磁共振功能成像在进展期直肠癌新辅助放化疗疗效评估中的应用[J]. 中国临床研究, 2020, 33(6): 759-63. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGCK202006008.htm [9] 董健, 李垣婕, 谢宗源, 等. 进展期直肠癌新辅助放化疗疗效评估模型的建立及评价: 基于MRI和网状蛋白1C[J]. 分子影像学杂志, 2021, 44(3): 472-7. doi: 10.12122/j.issn.1674-4500.2021.03.11 [10] Machackova T, Trachtova K, Prochazka V, et al. Tumor microRNAs identified by small RNA sequencing as potential response predictors in locally advanced rectal cancer patients treated with neoadjuvant chemoradiotherapy[J]. Cancer Genomics Proteomics, 2020, 17(3): 249-57. doi: 10.21873/cgp.20185 [11] Khalighfard S, Kalhori MR, Amiriani T, et al. A systematic approach introduced novel targets in rectal cancer by considering miRNA/ mRNA interactions in response to radiotherapy[J]. Cancer Biomark, 2022, 33(1): 97-110. doi: 10.3233/CBM-210079 [12] Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2018, 16(7): 874-901. doi: 10.6004/jnccn.2018.0061 [13] Kim NK, Hur H. New perspectives on predictive biomarkers of tumor response and their clinical application in preoperative chemoradiation therapy for rectal cancer[J]. Yonsei Med J, 2015, 56 (6): 1461-77. doi: 10.3349/ymj.2015.56.6.1461 [14] Molinari C, Matteucci F, Caroli P, et al. Biomarkers and molecular imaging as predictors of response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer[J]. Clin Colorectal Cancer, 2015, 14(4): 227-38. doi: 10.1016/j.clcc.2015.05.014 [15] 孙轶群, 刘宗霖, 付彩霞, 等. DKI直方图预测局部进展期直肠癌患者预后的临床价值[J]. 肿瘤影像学, 2022, 31(2): 105-12. https://www.cnki.com.cn/Article/CJFDTOTAL-YXYX202202001.htm [16] Li J, Zhang M, Wang CB. Circulating miRNAs as diagnostic biomarkers for multiple myeloma and monoclonal gammopathy of undetermined significance[J]. J Clin LabAnal, 2020, 34(6): e23233. [17] Tong T, Sun YQ, Gollub MJ, et al. Dynamic contrast-enhanced MRI: use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer[J]. J Magn Reson Imaging, 2015, 42(3): 673-80. doi: 10.1002/jmri.24835 [18] Zou HH, Yu J, Wei Y, et al. Response to neoadjuvant chemoradiotherapy for locally advanced rectum cancer: texture analysis of dynamic contrast-enhanced MRI[J]. J Magn Reson Imaging, 2019, 49(3): 885-93. doi: 10.1002/jmri.26254 [19] Nougaret S, Vargas HA, Lakhman Y, et al. Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses[J]. Radiology, 2016, 280(2): 446-54. doi: 10.1148/radiol.2016150702 [20] 肖楠, 陆艳荣, 朱丽娜, 等. DWI在直肠癌术前同步放化疗疗效预测中的作用[J]. 肿瘤防治研究, 2019, 46(4): 333-7. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLFY201904009.htm -







 下载:
下载: