Construction of a prediction model for predicting microvascular invasion in primary hepatocellular carcinoma
-
摘要:
目的 探讨术前基于影像学和血清学特征构建的列线图模型对肝癌微血管浸润(MVI)的预测价值。 方法 回顾性分析2015年1月~2020年12月于中山市人民医院接受切除或肝移植的548例肝细胞癌(HCC)患者的临床资料,最终纳入315例肝癌MVI患者,年龄53.2±11.5岁,肿瘤最大直径3.7~7.0 cm。收集患者临床及影像学资料并进行分析,采取单因素与多因素Logistic分析,筛查出能预测MVI的独立风险因素,构建预测HCC中MVI的列线图模型,利用ROC曲线、校准曲线和决策曲线对模型进行评估。 结果 MVI (+)患者的中位生存时间为13月(95%CI:8.1~17.9),1、3、5年无病生存率分别为50.6%、38.5%和30.9%(P < 0.05);MVI (-)患者的中位生存时间为47月(95%CI:32.7~61.3),1、3、5年无病生存率分别为77.9%、62.3%和38.8%(P < 0.05)。多因素Logistic回归分析显示,更大的肿瘤体积、突破肝外生长、缺乏或不完整假包膜、存在动脉期瘤周强化以及术前过高的球蛋白值是MVI (+)的独立危险因素(P < 0.05)。最终模型效能曲线下面积为0.895,95%CI为0.859-0.930,准确性为85.1%,敏感度为85.9%,特异性为84.1%。校准曲线显示预测概率与病理结果MVI (+)/MVI (-)概率有良好的一致性。决策曲线显示模型具有良好的临床应用价值。 结论 构建的列线图及预测模型能较好地术前预测MVI (+)的概率,可以根据MVI发生的风险调整HCC的治疗计划,以优化生存结果。 Abstract:Objective To investigate the value of a preoperative nomograph and prediction model based on imaging and serological characteristics for predicting microvascular invasion (MVI) in hepatocellular carcinoma (HCC). Methods Clinical data of 548 patients with HCC who underwent liver resection or liver transplantation from January 2015 to December 2020 in our Hospital were retrospectively included. A total of 315 patients with HCC (MVI+ or MVI-) with an average age of 53.2±11.5 years old and a maximum direct tumour of 3.7-7.0 cm were included. Clinical and imaging data were analyzed. Univariate and multivariate logistic analyses were used to screen out independent risk factors that could predict MVI, and a nomograph model was constructed to predict MVI in HCC, which was evaluated using a subject operating curve, calibration curve and decision curve. Results The median survival time of patients with MVI(+) was 13 months (95% CI: 8.1-17.9) while that of patients with MVI(-)was 47 months (95%CI: 32.7-61.3). The 1-year, 3-year and 5-year disease-free survival rates of patients with MVI(+) were 50.6%、38.5% and 30.9%, while that of patients with MVI(-) were 77.9%、62.3% and 38.8%, respectively. Multivariate logistic regression analysis showed that larger tumour size, extrahepatic growth, absence or incomplete pseudocapsule, presence of arterial peritumoral enhancement and high preoperative globulin value were independent risk factors for MVI(+). The final model efficacy were as follows: AUC=0.895, 95%CI: 0.859-0.930, accuracy: 85.1%, sensitivity: 85.9%, specificity: 84.1%. The calibration curve showed that the predicted probability was in good agreement with the MVI(+)/MVI(-) probability of the pathological results. Thus, the decision curve model displayed good clinical application value. Conclusion The constructed Nomograph and prediction model can better predict the probability of MVI(+) before surgery. It can aid in adjusting the treatment plan of HCC according to the risk of MVI to optimize the survival outcome. -
Key words:
- hepatocellular carcinoma /
- microvascular invasion /
- risk factors /
- nomograph
-
表 1 315例患者临床及影像基线特征
Table 1. Baseline clinical and imaging features of 315 patients[n(%)]
因素 总计(n=315) MVI(-)(n=145) MVI(+)(n=170) P 性別 0.579 女=0 42(13.3) 21(14.5) 21(12.4) 男=1 273(86.7) 124(85.5) 149(87.6) 年龄(岁, Mean±SD) 53.2±11.5 54.1±10.4 52.4±12.3 0.040 肿瘤最大直径(cm)* 4.9(3.7, 7.0) 3.7(2.6, 6.1) 5.9(3.6, 9) < 0.001 肿瘤边缘 0.665 光滑=0 64(20.3) 31(21.4) 33(19.4) 不光滑=1 251(79.7) 114(78.6) 137(80.6) 生长部位 0.001 肝内生长=0 202(64.1) 107(73.8) 95(55.9) 肝外生长=1 113(35.9) 38(26.2) 75(44.1) 累及肝段 0.001 一个肝段=0 127(40.3) 73(50.3) 54(31.8) 多个肝段=1 188(59.7) 72(49.7) 116(68.2) 肿瘤数量 0.968 单个=0 280(88.9) 129(89) 151(88.8) 多个=1 35(11.1) 16(11) 19(11.2) 假包膜 0.093 有=0 162(51.4) 82(56.6) 80(47.1) 无=1 153(48.6) 63(43.4) 90(52.9) 动脉期瘤周强化 < 0.001 无=0 128(40.6) 87(60) 41(24.1) 有=1 187(59.4) 58(40) 129(75.9) Stage分期 0.660 1=0 282(89.5) 131(90.3) 151(88.8) ≧2=1 33(10.5) 14(9.7) 19(11.2) 肝硬化 0.306 无=0 87(27.6) 36(24.8) 51(30) 有=1 228(72.4) 109(75.2) 119(70) 乙肝 0.091 无=0 59(18.7) 33(22.8) 26(15.3) 有=1 256(81.3) 112(77.2) 144(84.7) 术前谷丙转氨酶(U/L) 0.808 < 40=0 178(56.5) 83(57.2) 95(55.9) > 40=1 137(43.5) 62(42.8) 75(44.1) 术前谷草转氨酶(U/L) 0.046 < 35=0 146(46.3) 76(52.4) 70(41.2) > 35=1 169(53.7) 69(47.6) 100(58.8) AST/ALT 1(0.8, 1.3) 0.9(0.7, 1.2) 1(0.8, 1.4) 0.003 术前碱性磷酸酶(U/L) 0.215 < 150=0 284(90.2) 134(92.4) 150(88.2) > 150=1 31(9.8) 11(7.6) 20(11.8) 术前白蛋白(g/L) 0.293 < 55=0 95(30.2) 48(33.1) 47(27.6) > 55=1 220(69.8) 97(66.9) 123(72.4) 术前球蛋白(g/L) < 0.001 < 35=0 184(58.4) 125(86.2) 59(34.7) > 35=1 131(41.6) 20(13.8) 111(65.3) 白球比* 1.4(1.2, 1.6) 1.4(1.2, 1.6) 1.5(1.2, 1.7) 0.057 术前AFP(ng/mL) 0.150 < 20=0 121(38.4) 64(44.1) 57(33.5) 20~200=1 65(20.6) 28(19.3) 37(21.8) > 200=2 129(41.0) 53(36.6) 76(44.7) *以中位数(四分位数间距)表示.MVI: 微血管浸润. 表 2 临床影像实验室变量单因素和多因素Logistic回归分析
Table 2. Univariate and multivariate logistic regression analysis of clinical imaging laboratory variables
项目 单因素 多因素 OR (95%CI) P OR (95%CI) P 性別 女=0 Ref 男=1 1.202(0.627~2.302) 0.580 年龄 0.987(0.968~1.006) 0.183 肿瘤最大直径(cm) 1.231(1.138~1.332) < 0.001 1.237(1.116~1.372) < 0.001 肿瘤边缘 光滑=0 Ref 不光滑=1 1.129(0.652~1.956) 0.665 生长部位 肝内生长=0 Ref 肝外生长=1 2.223(1.378~3.586) 0.001 2.391(1.213~4.713) 0.012 累及肝段 一个肝段=0 Ref 多个肝段=1 2.178(1.377~3.445) 0.001 肿瘤数量 单个=0 Ref 多个=1 1.014(0.501~2.054) 0.968 假包膜 有=0 Ref 无1 1.464(0.938~2.286) 0.093 0.171(0.064~0.456) < 0.001 动脉期瘤周强化 无=0 Ref 有=1 4.72(2.91~7.655) < 0.001 20.618(7.504~56.65) < 0.001 Stage分期 1=0 Ref ≧2=1 1.177(0.568~2.441) 0.661 肝硬化 无=0 Ref 有=1 0.771(0.468~1.27) 0.307 乙肝 无=0 Ref 有=1 1.632(0.923~2.886) 0.092 术前谷丙转氨酶(U/L) < 40=0 Ref > 40=1 1.057(0.676~1.653) 0.808 术前谷草转氨酶(U/L) < 35=0 Ref > 35=1 1.573(1.007~2.46) 0.047 AST/ALT 1.621(1.065~2.469) 0.024 术前碱性磷酸酶(U/L) < 150=0 Ref > 150=1 1.624(0.751~3.514) 0.218 术前白蛋白(g/L) < 55=0 Ref > 55=1 1.295(0.8~2.098) 0.293 术前球蛋白(g/L) < 35=0 Ref > 35=1 11.758(6.664~20.749) < 0.001 17.077(8.531~34.185) < 0.001 白球比 0.889(0.695~1.136) 0.347 术前AFP(ng/mL) < 20=0 Ref 20~200=1 1.484(0.809~2.722) 0.203 > 200=2 1.61(0.976~2.656) 0.062 表 3 融合模型及单一变量对肝癌MVI的预测效能
Table 3. Prediction efficiency of fusion model and single variable for MVI of liver cancer
因素 AUC 敏感度(%) 特异性(%) 准确性(%) 融合模型 0.895 (0.859~0.930) 85.9 84.1 85.1 肿瘤最大直径 0.679 (0.621~0.738) 65.3 62.8 64.1 生长部位 0.590 (0.527~0.652) 44.1 73.8 57.8 假包膜 0.547 (0.484~0.611) 52.9 56.6 54.6 动脉期瘤周强化 0.679 (0.619~0.740) 75.9 60.0 68.6 术前球蛋白 0.758 (0.703~0.812) 65.3 86.2 74.9 注:构建融合模型的变量包括肿瘤最大直径、生长部位、假包膜、动脉期瘤周强化以及术前球蛋白. -
[1] Uemura T, Näppi JJ, Ryu Y, et al. A generative flow-based model for volumetric data augmentation in 3D deep learning for computed tomographic colonography[J]. Int J CARS, 2021, 16(1): 81-9. doi: 10.1007/s11548-020-02275-z [2] Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012 [J]. CAA Cancer J Clin, 2015, 65(2): 87-108. doi: 10.3322/caac.21262 [3] Zeng HM, Chen WQ, Zheng RS, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries[J]. Lancet Glob Health, 2018, 6(5): e555-67. doi: 10.1016/S2214-109X(18)30127-X [4] Vitale A, Peck-Radosavljevic M, Giannini EG, et al. Personalized treatment of patients with very early hepatocellular carcinoma[J]. J Hepatol, 2017, 66(2): 412-23. doi: 10.1016/j.jhep.2016.09.012 [5] Zhang XP, Chen ZH, Zhou TF, et al. A nomogram to predict early postoperative recurrence of hepatocellular carcinoma with portal vein tumour thrombus after R0 liver resection: a large-scale, multicenter study[J]. Eur J Surg Oncol, 2019, 45(9): 1644-51. doi: 10.1016/j.ejso.2019.03.043 [6] Pan YX, Chen JC, Fang AP, et al. A nomogram predicting the recurrence of hepatocellular carcinoma in patients after laparoscopic hepatectomy[J]. Cancer Commun (Lond), 2019, 39(1): 55. doi: 10.1186/s40880-019-0404-6 [7] Lee S, Kang TW, Song KD, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation[J]. Ann Surg, 2021, 273(3): 564-71. doi: 10.1097/SLA.0000000000003268 [8] Qi YP, Zhong JH, Liang ZY, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion[J]. Am J Surg, 2019, 217(4): 739-44. doi: 10.1016/j.amjsurg.2018.07.054 [9] Song T, Wang C, Guo C, et al. Pentraxin 3 overexpression accelerated tumor metastasis and indicated poor prognosis in hepatocellular carcinoma via driving epithelial-mesenchymal transition[J]. J Cancer, 2018, 9(15): 2650-8. doi: 10.7150/jca.25188 [10] Lim KC, Chow PKH, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria[J]. Ann Surg, 2011, 254(1): 108-13. doi: 10.1097/SLA.0b013e31821ad884 [11] Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis[J]. Lancet Oncol, 2009, 10(1): 35-43. doi: 10.1016/S1470-2045(08)70284-5 [12] Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial[J]. Ann Surg, 2007, 245(1): 36-43. doi: 10.1097/01.sla.0000231758.07868.71 [13] PhD MRP, Md TVL, Md LA, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability[J]. Ann Surg Oncol, 2013, 20(1): 325-39. doi: 10.1245/s10434-012-2513-1 [14] Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more personalized approach to cancer staging [J]. CA Cancer J Clin, 2017, 67(2): 93-9. doi: 10.3322/caac.21388 [15] Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma[J]. Gastroenterology, 2009, 137(3): 850-5. doi: 10.1053/j.gastro.2009.06.003 [16] Zhao WC, Fan LF, Yang N, et al. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma [J]. Eur J Surg Oncol EJSO, 2013, 39(8): 858-64. doi: 10.1016/j.ejso.2013.04.003 [17] Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-Ⅱ for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion[J]. J Hepatol, 2015, 62(4): 848-54. doi: 10.1016/j.jhep.2014.11.005 [18] Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine[J]. Nat Rev Clin Oncol, 2017, 14(12): 749-62. doi: 10.1038/nrclinonc.2017.141 [19] Xu X, Zhang HL, Liu QP, et al. Radiomic analysis of contrastenhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma[J]. J Hepatol, 2019, 70(6): 1133-44. doi: 10.1016/j.jhep.2019.02.023 [20] Yang L, Gu DS, Wei JW, et al. A radiomics nomogram for preoperative prediction of microvascular invasion in hepatocellular carcinoma [J]. Liver Cancer, 2019, 8(5): 373-86. doi: 10.1159/000494099 [21] Lee S, Kim SH, Lee JE, et al. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma[J]. J Hepatol, 2017, 67(3): 526-34. doi: 10.1016/j.jhep.2017.04.024 [22] Zhang YF, Lei XZ, Xu LL, et al. Preoperative and postoperative nomograms for predicting early recurrence of hepatocellular carcinoma without macrovascular invasion after curative resection [J]. BMC Surg, 2022, 22(1): 233. doi: 10.1186/s12893-022-01682-0 [23] Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality[J]. Hepatology, 2015, 61(1): 191-9. doi: 10.1002/hep.27388 [24] Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma[J]. Semin Liver Dis, 2005, 25(2): 181-200. doi: 10.1055/s-2005-871198 [25] Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis[J]. Ann Surg, 2015, 261(5): 947-55. doi: 10.1097/SLA.0000000000000710 [26] Lee KW, Park JW, Park JB, et al. Liver transplantation for hepatocellular carcinoma with bile duct thrombi[J]. Transplant Proc, 2006, 38(7): 2093-4. doi: 10.1016/j.transproceed.2006.06.034 [27] Han J, Li ZL, Xing H, et al. The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: a multi-institutional study[J]. HPB, 2019, 21(8): 962-71. doi: 10.1016/j.hpb.2018.11.005 [28] Roayaie S, Obeidat K, Sposito C, et al. Resection of hepatocellular cancer ≤2 cm: results from two Western centers[J]. Hepatology, 2013, 57(4): 1426-35. doi: 10.1002/hep.25832 [29] Yo-ichi Yamashita MD P, Eiji Tsuijita MD P, Md KT, et al. Predictors for microinvasion of small hepatocellular carcinoma ≤2 cm[J]. Ann Surg Oncol, 2012, 19(6): 2027-34. doi: 10.1245/s10434-011-2195-0 [30] Cuccurullo V, di Stasio GD, Mazzarella G, et al. Microvascular invasion in HCC: the molecular imaging perspective[J]. Contrast Media Mol Imaging, 2018, 2018: 9487938. [31] Sun Z, Li Z, Shi XL, et al. Anatomic versus non-anatomic resection of hepatocellular carcinoma with microvascular invasion: a systematic review and meta-analysis[J]. Asian J Surg, 2021, 44(9): 1143-50. doi: 10.1016/j.asjsur.2021.02.023 [32] Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma[J]. Liver Transplant, 2005, 11(9): 1086-92. doi: 10.1002/lt.20472 [33] Md SS, Md ON, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma [J]. Ann Surg Oncol, 2014, 21(3): 1002-9. doi: 10.1245/s10434-013-3376-9 [34] Iguchi T, Shirabe K, Aishima S, et al. New pathologic stratification of microvascular invasion in hepatocellular carcinoma[J]. Transplantation, 2015, 99(6): 1236-42. doi: 10.1097/TP.0000000000000489 [35] Jeong SH, Kim RB, Park SY, et al. Nomogram for predicting gastric cancer recurrence using biomarker gene expression[J]. Eur J Surg Oncol, 2020, 46(1): 195-201. doi: 10.1016/j.ejso.2019.09.143 [36] Yin XY, Pang T, Liu Y, et al. Development and validation of a nomogram for preoperative prediction of lymph node metastasis in early gastric cancer[J]. World J Surg Oncol, 2020, 18(1): 2. doi: 10.1186/s12957-019-1778-2 [37] Wang HH, Lu Y, Liu RK, et al. A non-invasive nomogram for preoperative prediction of microvascular invasion risk in hepatocellular carcinoma[J]. Front Oncol, 2021, 11((5)): 745085. [38] Li HG, Li T, Hu JH, et al. A nomogram to predict microvascular invasion in early hepatocellular carcinoma[J]. J Cancer Res Ther, 2021, 17(3): 652-7. doi: 10.4103/jcrt.JCRT_1714_20 [39] Gu YF, Zheng FY, Zhang YX, et al. Novel nomogram based on inflammatory markers for the preoperative prediction of microvascular invasion in solitary primary hepatocellular carcinoma [J]. Cancer Manag Res, 2022, 14: 895-907. doi: 10.2147/CMAR.S346976 -
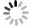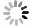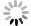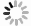






 下载:
下载: