Application of magnetic resonance NODDI technique on hippocampal microstructure in patients with Parkinson's cognitive impairment
-
摘要:
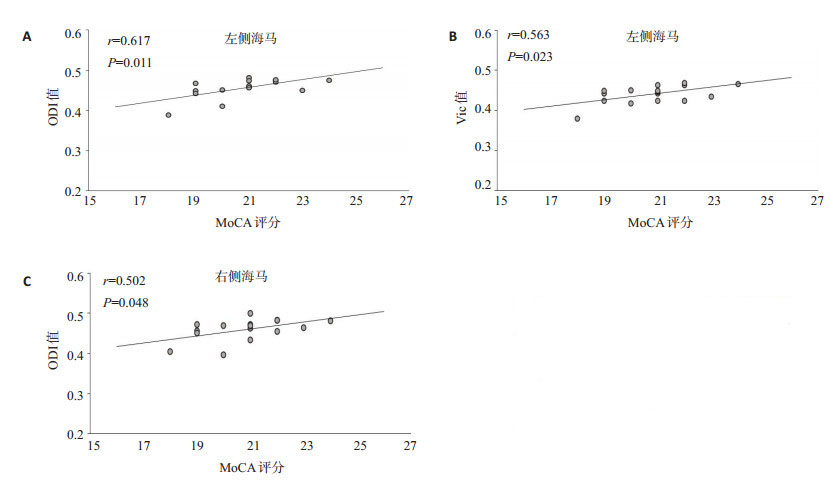
目的 利用神经突方向离散度与密度成像(NODDI)探索帕金森认知障碍(PD-CI)患者海马微观结构的变化,并探讨其与认知功能的关系。 方法 选择我院36例帕金森病患者为帕金森病组,选取20例健康志愿者为对照组,对两组进行磁共振检查,检查前对PD患者进行蒙特利尔认知评分(MoCA),其中16例MoCA评分 < 26分为PD-CI组,20例MoCA评分≥26分为帕金森认知正常组。根据NODDI扫描结果获取相关参数:方向离散度指数(ODI)、细胞内体积分数(Vic)和各向同性水分子体积分数(Viso)值,分析感兴趣区ODI、Vic及Viso值的组间差异。将差异有统计学意义的指标分别与MoCA量表评分进行Pearson相关分析,研究各扩散指数与MoCA量表得分的相关性。 结果 PD患者海马的ODI值和Vic值均低于健康对照组(P < 0.05),且PD-CI组的ODI值和Vic值低于帕金森认知正常组,差异有统计学意义(P < 0.05),而Viso值与健康对照组相比差异无统计学意义。采用Pearson相关分析对海马ODI值和Vic值与MoCA量表评分进行相关性分析,得出左侧海马ODI值与MoCA量表评分相关性最强(r=0.617,P < 0.05)。 结论 NODDI技术能够反映PD患者海马微观结构的改变,并在一定程度上体现了患者的临床认知状态,从而在其辅助诊断中提供重要的参考价值。 -
关键词:
- 帕金森病 /
- 认知功能障碍 /
- 海马 /
- 神经突方向离散度与密度成像
Abstract:Objective To explore the changes of hippocampal microstructure in patients with Parkinson's disease cognitive impairment (PD-CI) by using neurite orientation dispersion and density imaging (NODDI), and explore its relationship with cognitive function. Methods Thirty-six patients with Parkinson's disease in our hospital were selected as Parkinson's disease group, and 20 healthy volunteers were selected as control group. The patients with PD were given Montreal Cognitive Score (MoCA) before examination. Among them, 16 patients with MoCA score < 26 were divided into PD-CI group (PD-CI), and 20 patients with MoCA score ≥26 were divided into normal Parkinson's cognition group. According to the results of NODDI scanning, the related parameters, such as orientation dispersion index (ODI), intracellular volume fraction (Vic) and isotropic water molecule volume fraction (Viso) were obtained, and the differences in ODI, Vic and Viso values in the region of interest were analyzed. The indicators with statistically significant differences were each subjected to Pearson correlation analysis with MoCA scale scores to investigate the correlation between each diffusion index and MoCA scale scores. Results The values of ODI and Vic in hippocampus of PD patients were significantly lower than those in healthy control group (P < 0.05), and the values of ODI and Vic in PD-CI group were lower than those in cognitive normal of Parkinson's disease group, with a statistically significant difference (P < 0.05), but there was no significant difference between Viso and healthy control group. The left hippocampal ODI value correlated most strongly with the MoCA scale score (r=0.617, P < 0.05). Conclusion NODDI technique can reflect the changes of hippocampal microstructure of PD patients, and to a certain extent, reflecting the clinical cognitive status of PD patients, thus providing important reference value in its auxiliary diagnosis. -
表 1 3组受试者一般情况及MoCA量表评分分析
Table 1. General situation of three groups of subjects and score analysis of MoCA scale
因素 PD-CI (n=16) PD-CN (n=20) NC (n=20) P 性别(男/女) 10/6 10/10 12/8 0.715 年龄(岁) 61.88±6.27 61.45±7.63 60.95±6.35 0.921 MoCA评分 20.81±1.60ab 27.20±1.06 28.45±1.15 < 0.001 aP < 0.05 vs PD-CN组; bP < 0.05 vs NC组; PD-CI: 帕金森认知障碍组; PD-CN:帕金森认知正常组; NC: 对照组. 表 2 PD组与对照组海马各参数指标的均值
Table 2. Mean values of parameters of hippocampus between PD group and control group (Mean±SD)
参数 PD-CI PD-CN NC ODI 左侧 0.456±0.025 0.473±0.019 0.489±0.015 右侧 0.459±0.028 0.479±0.020 0.496±0.015 Vic 左侧 0.441±0.023 0.460±0.019 0.481±0.023 右侧 0.446±0.015 0.462±0.019 0.479±0.023 Viso 左侧 0.109±0.031 0.097±0.027 0.086±0.028 右侧 0.106±0.016 0.098±0.021 0.092±0.014 ODI: 方向离散度指数; Vic: 细胞内体积分数; Viso: 各向同性水分子体积分数. 表 3 双侧海马各参数指标的组间比较
Table 3. Comparison of parameters of bilateral hippocampus between groups
参数 组别 组别 P(左侧) P(右侧) ODI PD-CI PD-CN 0.043 0.022 NC < 0.001 < 0.001 PD-CN PD-CI 0.043 0.022 NC 0.043 0.028 Vic PD-CI PD-CN 0.032 0.044 NC < 0.001 < 0.001 PD-CN PD-CI 0.032 0.044 NC 0.010 0.033 Viso PD-CI PD-CN 0.644 0.554 NC 0.056 0.060 PD-CN PD-CI 0.644 0.554 NC 0.662 0.805 表 4 PD-CI组ODI值和Vic值与MoCA量表评分的相关性分析
Table 4. Correlation analysis of ODI value, Vic value and MoCA scale score in PD-CI group
指标 ODI(左) Vic(左) ODI(右) Vic(右) r 0.617 0.563 0.502 0.312 P 0.011 0.023 0.048 0.240 -
[1] Khoo TK, Yarnall AJ, Duncan GW, et al. The spectrum of nonmotor symptoms in early Parkinson disease[J]. Neurology, 2013, 80(3): 276-81. doi: 10.1212/WNL.0b013e31827deb74 [2] Aarsland D, Creese B, Politis M, et al. Cognitive decline in Parkinson disease[J]. Nat Rev Neurol, 2017, 13(4): 217-31. [3] Saghafi S, Ferguson L, Hogue O, et al. Histopathologic subtype of hippocampal sclerosis and episodic memory performance before and after temporal lobectomy for epilepsy[J]. Epilepsia, 2018, 59 (4): 825-33. doi: 10.1111/epi.14036 [4] Tezer FI, Xasiyev F, Soylemezoglu F, et al. Clinical and electrophysiological findings in mesial temporal lobe epilepsy with hippocampal sclerosis, based on the recent histopathological classifications[J]. Epilepsy Res, 2016, 127: 50-4. doi: 10.1016/j.eplepsyres.2016.08.012 [5] Diederich K, Bastl A, Wersching H, et al. Effects of different exercise strategies and intensities on memory performance and neurogenesis [J]. Front Behav Neurosci, 2017, 11: 47. [6] Győrfi O, Nagy H, Bokor M, et al. Insights into the structure and function of the hippocampal formation: relevance to Parkinson's disease[J]. Ideggyogy Sz, 2018: 15-24. doi: 10.18071/isz.71.0015 [7] Schneider CB, Donix M, Linse K, et al. Accelerated age-dependent hippocampal volume loss in parkinson disease with mild cognitive impairment[J]. Am J Alzheimers Dis Other Demen, 2017, 32(6): 313-9. doi: 10.1177/1533317517698794 [8] Zhang H, Schneider T, Wheeler- Kingshott CA, et al. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain[J]. NeuroImage, 2012, 61(4): 1000-16. doi: 10.1016/j.neuroimage.2012.03.072 [9] Radhakrishnan H, Stark SM, Stark CEL. Microstructural alterations in hippocampal subfields mediate age-related memory decline in humans[J]. Front Aging Neurosci, 2020, 12(1): 94. [10] 刘伟星, 陆鹏, 张晓斌, 等. 磁共振神经突方向离散度与密度成像对帕金森病患者壳核病变的临床应用[J]. 磁共振成像, 2020, 11(8): 610-4. https://www.cnki.com.cn/Article/CJFDTOTAL-CGZC202008005.htm [11] Merluzzi AP, Dean DC Ⅲ, Adluru N Ⅲ, et al. Age-dependent differences in brain tissue microstructure assessed with neurite orientation dispersion and density imaging[J]. Neurobiol Aging, 2016, 43: 79-88. doi: 10.1016/j.neurobiolaging.2016.03.026 [12] Becker S, Granert O, Timmers M, et al. Association of hippocampal subfields, CSF biomarkers, and cognition in patients with parkinson disease without dementia[J]. Neurology, 2021, 96(6): e904-15. [13] Chen YS, Chen HL, Lu CH, et al. The corticolimbic structural covariance network as an early predictive biosignature for cognitive impairment in Parkinson's disease[J]. Sci Rep, 2021, 11(1): 1-9. doi: 10.1038/s41598-020-79139-8 [14] 陈旭辉, 林志坚, 吴军, 等. 弥散张量成像测量海马体各向异性分数在帕金森病认知障碍中的应用[J]. 中风与神经疾病杂志, 2019, 36 (1): 7-9. https://www.cnki.com.cn/Article/CJFDTOTAL-ZFSJ201901002.htm [15] Andica C, Kamagata K, Hatano T, et al. Neurocognitive and psychiatric disorders-related axonal degeneration in Parkinson's disease[J]. J Neurosci Res, 2020, 98(5): 936-49. doi: 10.1002/jnr.24584 [16] Chen FX, Kang DZ, Chen FY, et al. Gray matter atrophy associated with mild cognitive impairment in Parkinson's disease[J]. Neurosci Lett, 2016, 617: 160-5. doi: 10.1016/j.neulet.2015.12.055 [17] Filippi M, Canu E, Donzuso G, et al. Tracking cortical changes throughout cognitive decline in Parkinson's disease[J]. Mov Disord, 2020, 35(11): 1987-98. doi: 10.1002/mds.28228 [18] Lim Y, Kehm VM, Lee EB, et al. -syn suppression reverses synaptic and memory defects in a mouse model of dementia with lewy bodies[J]. J Neurosci, 2011, 31(27): 10076-87. doi: 10.1523/JNEUROSCI.0618-11.2011 [19] Longhena F, Faustini G, Varanita T, et al. Synapsin Ⅲ is a key component of α- synuclein fibrils in Lewy bodies of PD brains[J]. Brain Pathol, 2018, 28(6): 875-88. doi: 10.1111/bpa.12587 [20] 马东辉, 刘存存, 黄小盼, 等. 磁共振轴突定向弥散和密度成像技术评估帕金森患者小脑微结构变化[J]. 分子影像学杂志, 2021, 44(1): 22-6. doi: 10.12122/j.issn.1674-4500.2021.01.04 -







 下载:
下载: