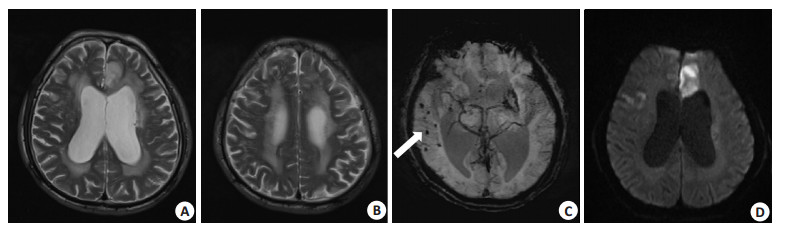
Cerebral small vessel disease load predicts poor prognosis in mild acute cerebral infarction after intravenous thrombolysis
-
摘要:
目的 探讨脑小血管病(CSVD)负荷与轻型急性脑梗死(ACI)静脉溶栓后3月不良预后的关系。 方法 回顾性分析2016~2019年在东莞市人民医院神经内科接受重组组织纤溶酶原激活剂静脉溶栓的161例轻型ACI患者的资料。研究的临床变量包括年龄、性别、血管危险因素、美国国立卫生研究院卒中评分(NIHSS)及血液学和MR参数。轻型ACI定义为基线NIHSS≤7分,3月不良预后定义为改良Rankin评分≥3分。CSVD负荷包括脑白质高信号(WMHs)、腔隙性脑梗死、脑微出血(CMBs)和扩大的周围血管间隙。根据头颅MR影像评价CSVD总体负荷,根据Fazekas分级量表评估WMHs严重程度。 结果 161例患者中男性117例(72.7%),3月不良预后患者为29例(18.0%)。单因素分析显示,基线NIHSS、房颤、症状性颅内动脉狭窄、WMHs和CMBs与急性轻型ACI静脉溶栓后3月不良预后有关(P < 0.05)。分别将WMHs及CMBs进入两个Logistic回归方程模型。模型1中,基线NIHSS(OR=1.601, 95% CI: 1.203~2.130, P=0.001)、症状性颅内动脉狭窄(OR=2.658, 95% CI: 1.013~6.978, P=0.047)和WMHs(OR=1.449, 95% CI: 1.033~2.031, P=0.032)与轻型ACI静脉溶栓后3月不良预后显著相关;模型2中,基线NIHSS(OR=1.650, 95% CI: 1.232~2.210, P=0.001),症状性颅内动脉狭窄(OR=3.732, 95% CI=1.435~9.702, P=0.007)和CMBs(OR=1.242, 95% CI: 1.062~1.452, P=0.007)与轻型ACI静脉溶栓后3月不良预后显著相关。 结论 WMHs和CMBs是轻型ACI静脉溶栓后3月不良预后的预测因子。 Abstract:Objective To investigate the relationship between cerebral small vessel disease (CSVD) load and 3-month poor prognosis in mild acute cerebral infarction (ACI) treated with recombinant tissue plasminogen activator. Methods 161 patients with mild ACI who received intravenous thrombolysis with recombinant tissue plasminogen activator from 2016 to 2019 were retrospectively analyzed. Clinical variables studied included age, gender, vascular risk factors, National Institute of Health Stroke Score (NIHSS) as well as initial hematologic and MR parameters. Mild acute cerebral infarction was defined as a baseline NIHSS score ≤7 and a 3-month poor prognosis was defined as a modified Rankin score ≥3. CSVD load included white matter hyperintensities (WMHs), lacunes, cerebral microbleeds (CMBs) and enlarged perivascular space. Total CSVD load was evaluated based on the clinical MR images, and severity WMHs was assessed according to the Fazekas criteria. Results Of the 161 patients, 117 (72.7%) were male, and the number of patients with a poor 3-month prognosis was 29 (18.0%). Univariate analysis showed that baseline NIHSS, atrial fibrillation, symptomatic intracranial artery stenosis (SIAS), WMHs and CMBs were correlated with 3-month poor prognosis (P < 0.05). WMHs and CMBs were entered into two logistic regression equation models, respectively. In Model 1, baseline NIHSS (OR=1.601, 95% CI: 1.203-2.130, P=0.001), symptomatic intracranial artery stenosis (OR=2.658, 95% CI: 1.013-6.978, P=0.047) and WMHs (OR=1.449, 95% CI: 1.033-2.031, P=0.032) were significantly associated with 3-month poor prognosis. In Model 2, baseline NIHSS (OR=1.650, 95% CI: 1.232- 2.210, P=0.001), symptomatic intracranial artery stenosis (OR=3.732, 95% CI: 1.435-9.702, P=0.007) and CMBs (OR=1.242, 95% CI: 1.062-1.452, P=0.007) were significantly correlated with poor prognosis at 3 months after intravenous thrombolysis for mild ACI. Conclusion WMHs and CMBs are predictors of poor prognosis at 3 months after intravenous thrombolysis in mild ACI. -
表 1 人口学与临床特征
Table 1. Demographic and clinical characteristics of the study sample (n=161)
指标 值 年龄(岁, Mean±SD) 60.8±11.6 男性[n (%)] 117 (72.7) 高血压病史[n (%)] 123 (76.4) 糖尿病病史[n (%)] 48 (29.8) 吸烟史[n (%)] 62 (38.5) 房颤[n (%)] 28 (17.4) 缺血性卒中史[n (%)] 26 (16.1) 抗血小板药使用史[n (%)] 21 (13.0) 基线NIHSS 4 (3~7) 入院血糖(mmol/L, Mean±SD) 7.7±3.2 症状性颅内动脉狭窄[n (%)] 38 (23.6) 腔隙性脑梗死[n (%)] 67(41.6) WMHs分级 0 (0~1) CMBs数 0 (0~1) EPVS分级 0 (0~1) 总体CSVD负荷 1 (0~2) 不良预后[n (%)] 29(18.0) NIHSS: 美国国立卫生研究院卒中评分; WMHs: 脑白质高信号; CMBs: 脑微出血; EPVS: 扩大的血管周围间隙; CSVD: 脑小血管病. 表 2 轻型ACI静脉溶栓后3月不良预后危险因素的单因素分析
Table 2. Risk factors of 3-month poor outcome in univariable analysis
指标 不良预后组(n=29) 良好预后组(n=132) t/χ2/Z P 年龄(岁, Mean±SD)a 62.9±10.5 60.3±11.8 -1.110 0.132 男性[n (%)]b 19 (65.5) 98 (74.2) 0.911 0.340 高血压病史[n (%)]b 22 (75.9) 101 (76.5) 0.006 0.940 糖尿病病史[n (%)]b 7 (24.1) 41(31.1) 0.545 0.461 吸烟史[n (%)]b 10 (34.5) 52 (39.4) 0.242 0.623 房颤[n (%)]b 9 (31.0) 19 (14.4) 4.583 0.032 缺血性卒中史[n (%)]b 7 (24.1) 19 (14.4) 1.667 0.197 抗血小板药使用史[n (%)]b 6 (20.7) 15 (11.4) 1.823 0.177 基线NIHSSc 6 (5~7) 4 (3~6) -3.763 < 0.001 入院血糖(mmol/L, Mean±SD)a 7.3 (2.4) 7.8 (3.4) 0.757 0.156 症状性颅内动脉狭窄[n (%)]b 13 (44.8) 25 (18.9) 8.837 0.003 腔隙性脑梗死[n (%)]b 16 (55.2) 51 (38.6) 2.676 0.102 WMHs分级c 1 (0~3.5) 0 (0~1) -2.017 0.044 CMBs数c 0 (0~3) 0 (0~1) -2.072 0.038 EPVS分级c 1 (1~1) 1 (0~1) -1.879 0.060 总体CSVD负荷c 1 (0~2) 1 (0~1) 0.114 0.114 a组间比较行t检验; b组间比较行卡方检验; c组间比较行秩和检验. 表 3 轻型ACI静脉溶栓后3月不良预后危险因素的Logistic回归分析
Table 3. Multivariate logistic regression of predictors for 3-month poor outcome
变量 β OR(95% CI) P 模型1 基线NIHSS 0.470 1.601 (1.203~2.130) 0.001 房颤 0.860 2.363 (0.794~7.032) 0.122 症状性颅内动脉狭窄 0.978 2.658 (1.013~6.978) 0.047 WMHs 0.371 1.499 (1.033~2.031) 0.032 模型2 基线NIHSS 0.501 1.650 (1.232~2.210) 0.001 房颤 0.756 2.129 (0.735~6.169) 0.164 症状性颅内动脉狭窄 1.317 3.732 (1.435~9.702) 0.007 CMBs数 0.217 1.242 (1.062~1.452) 0.007 -
[1] Collaborators GBD2S. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016[J]. Lancet Neurol, 2019, 18(5): 439-58. doi: 10.1016/S1474-4422(19)30034-1 [2] Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke[J]. J Vasc Surg, 2008, 48 (6): 1634-5. [3] The national institute of neurological disorders and stroke rt-pa stroke study group. tissue plasminogen activator for acute ischemic stroke[J]. New Engl J Med, 1995, 24(333): 1581-7. [4] Dhamoon MS, Moon YP, Paik MC, et al. Long-term functional recovery after first ischemic stroke[J]. Stroke, 2009, 40(8): 2805-11. doi: 10.1161/STROKEAHA.109.549576 [5] Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials[J]. Lancet, 2014, 384(9958): 1929-35. doi: 10.1016/S0140-6736(14)60584-5 [6] Yu AYX, Hill MD, Coutts SB. Should minor stroke patients be thrombolyzed? A focused review and future directions[J]. Int J Stroke, 2015, 10(3): 292-7. doi: 10.1111/ijs.12426 [7] Romano JG, Smith EE, Liang L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis[J]. JAMA Neurol, 2015, 72(4): 423. doi: 10.1001/jamaneurol.2014.4354 [8] Liu YL, Yin HP, Qiu DH, et al. Multiple hypointense vessels on susceptibility-weighted imaging predict early neurological deterioration in acute ischaemic stroke patients with severe intracranial large artery Stenosis or occlusion receiving intravenous thrombolysis[J]. Stroke Vasc Neurol, 2020, 5(4): 361-7. doi: 10.1136/svn-2020-000343 [9] Yu WM, Abdul-Rahim AH, Cameron AC, et al. The incidence and associated factors of early neurological deterioration after thrombolysis[J]. Stroke, 2020, 51(9): 2705-14. doi: 10.1161/STROKEAHA.119.028287 [10] Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging[J]. Lancet Neurol, 2013, 12(5): 483-97. doi: 10.1016/S1474-4422(13)70060-7 [11] Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges[J]. Lancet Neurol, 2010, 9(7): 689-701. doi: 10.1016/S1474-4422(10)70104-6 [12] Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration[J]. Lancet Neurol, 2013, 12(8): 822-38. doi: 10.1016/S1474-4422(13)70124-8 [13] Huo YC, Li Q, Zhang WY, et al. Total small vessel disease burden predicts functional outcome in patients with acute ischemic stroke[J]. Front Neurol, 2019, 10: 808. doi: 10.3389/fneur.2019.00808 [14] Sagnier S, Catheline G, Dilharreguy B, et al. Normal- appearing white matter integrity is a predictor of outcome after ischemic stroke[J]. Stroke, 2020, 51(2): 449-56. doi: 10.1161/STROKEAHA.119.026886 [15] Ryu WS, Woo SH, Schellingerhout D, et al. Stroke outcomes are worse with larger leukoaraiosis volumes[J]. Brain, 2017, 140(1): 158-70. doi: 10.1093/brain/aww259 [16] Jokinen H, Koikkalainen J, Laakso HM, et al. Global burden of small vessel disease- related brain changes on MRI predicts cognitive and functional decline[J]. Stroke, 2020, 51(1): 170-8. doi: 10.1161/STROKEAHA.119.026170 [17] Arba F, Palumbo V, Boulanger JM, et al. Leukoaraiosis and lacunes are associated with poor clinical outcomes in ischemic stroke patients treated with intravenous thrombolysis[J]. Int J Stroke, 2016, 11(1): 62-7. doi: 10.1177/1747493015607517 [18] Liu YY, Zhang M, Chen Y, et al. The degree of leukoaraiosis predicts clinical outcomes and prognosis in patients with middle cerebral artery occlusion after intravenous thrombolysis[J]. Brain Res, 2018, 1681: 28-33. doi: 10.1016/j.brainres.2017.12.033 [19] Charidimou A, Turc G, Oppenheim C, et al. Microbleeds, Cerebral Hemorrhage, and functional outcome after stroke thrombolysis[J]. Stroke, 2017, 48(8): 2084-90. doi: 10.1161/STROKEAHA.116.012992 [20] 王琳, 赵小媛, 江俊莹, 等. 脑白质高信号而非脑小血管病总体负担与急性缺血性卒中患者转归相关[J]. 国际脑血管病杂志, 2020, 28 (6): 420-5. doi: 10.3760/cma.j.issn.1673-4165.2020.06.004 [21] Powers WJ, Rabinstein AA. Response by Powers and rabinstein to letter regarding article, "2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association"[J]. Stroke, 2019, 50(9): e46-e110. [22] Campbell BCV. Challenges of mild stroke[J]. Stroke, 2020, 51(11): 3203-4. doi: 10.1161/STROKEAHA.120.032358 [23] Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS[J]. Kardiol Pol, 2016, 74(12): 1359-469. doi: 10.5603/KP.2016.0172 [24] Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging[J]. Am J Roentgenol, 1987, 149(2): 351-6. doi: 10.2214/ajr.149.2.351 [25] Staals J, Makin SDJ, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden[J]. Neurology, 2014, 83(14): 1228-34. doi: 10.1212/WNL.0000000000000837 [26] Doubal FN, MacLullich AMJ, Ferguson KJ, et al. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease[J]. Stroke, 2010, 41(3): 450-4. doi: 10.1161/STROKEAHA.109.564914 [27] Chimowitz M, Strong J, Brown M, et al. Prognosis of patients with symptomatic vertebral or basilar artery stenosis[J]. Stroke, 1998, 29 (7): 1389-92. doi: 10.1161/01.STR.29.7.1389 [28] Palumbo V, Boulanger JM, Hill MD, et al. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke[J]. Neurology, 2007, 68(13): 1020-4. doi: 10.1212/01.wnl.0000257817.29883.48 [29] Schmidt R, Seiler S, Loitfelder M. Longitudinal change of small-vessel disease-related brain abnormalities[J]. J Cereb Blood Flow Metab, 2016, 36(1): 26-39. doi: 10.1038/jcbfm.2015.72 [30] Dey AK, Stamenova V, Turner G, et al. Pathoconnectomics of cognitive impairment in small vessel disease: a systematic review[J]. Alzheimer's Dement, 2016, 12(7): 831-45. doi: 10.1016/j.jalz.2016.01.007 [31] Zhang M, Zhu WS, Yun WW, et al. Correlation of matrix metalloproteinase-2 single nucleotide polymorphisms with the risk of small vessel disease (SVD)[J]. J Neurol Sci, 2015, 356(1/2): 61-4. [32] Simão F, Ustunkaya T, Clermont AC, et al. Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke[J]. Blood, 2017, 129(16): 2280-90. doi: 10.1182/blood-2016-09-740670 [33] Valdés Hernández MDC, Booth T, Murray C, et al. Brain white matter damage in aging and cognitive ability in youth and older age [J]. Neurobiol Aging, 2013, 34(12): 2740-7. doi: 10.1016/j.neurobiolaging.2013.05.032 [34] Wilson D, Charidimou A, Ambler G, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA[J]. Neurology, 2016, 87(14): 1501-10. doi: 10.1212/WNL.0000000000003183 [35] Zand R, Shahjouei S, Tsivgoulis G, et al. Cerebral microbleeds are associated with higher mortality among ischemic stroke patients[J]. J Stroke Cerebrovasc Dis, 2018, 27(11): 3036-42. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.037 [36] Ding J, Sigurðsson S, Jónsson PV, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community[J]. Neurology, 2017, 88(22): 2089-97. doi: 10.1212/WNL.0000000000003983 [37] Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy- related microbleeds[J]. AJNR Am J Neuroradiol, 1999, 20(4): 637-42. [38] Cordonnier C. Brain microbleeds: more evidence, but still a clinical dilemma[J]. Curr Opin Neurol, 2011, 24(1): 69-74. doi: 10.1097/WCO.0b013e328341f8c0 [39] Xiao WM, Chen YK, Li W, et al. Microbleeds in Fronto-subcortical circuits are predictive of dementia conversion in patients with vascular cognitive impairment but no dementia[J]. Neural Regen Res, 2018, 13(11): 1913. doi: 10.4103/1673-5374.239441 [40] D'Souza CE, Greenway MRF, Graff-Radford J, et al. Cognitive impairment in patients with stroke[J]. Semin Neurol, 2021, 41(1): 75-84. doi: 10.1055/s-0040-1722217 [41] Sakuta K, Yaguchi H, Nakada R, et al. Cerebral microbleeds load and long-term outcomes in minor ischemic stroke[J]. J Stroke Cerebrovasc Dis, 2021, 30(9): 105973. doi: 10.1016/j.jstrokecerebrovasdis.2021.105973 -







 下载:
下载: