Epidemiological and clinical characteristics of patient with COVID-19
-
摘要:
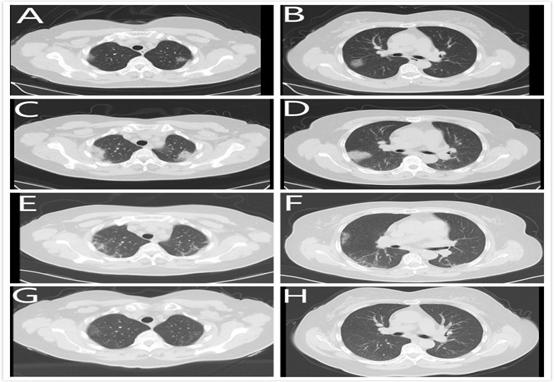
目的回顾性分析临沂市COVID-19患者的流行病学和临床特征,描述COVID-19的发生与演变过程,为防控策略提供科学依据。 方法选取临沂市41例COVID-19患者为研究对象,其中轻型9例,普通型25例,重型及危重型7例,收集和分析患者的流行病学和临床特征数据。 结果患者的中位年龄为41岁(31.6~53.6岁),男女比例1.56:1,中位体质量指数25.1 kg/m(2 22.4~ 27.6 kg/m2),重型及危重型患者年龄更大、体质量更大、合并更多的基础疾病。有湖北旅居史者15例(36.6%),聚集性发病患者33例(80.5%),中位潜伏期6.5 d(4.0~11.5 d)。常见症状包括发热27例(65.9%)、干咳32例(78.0%),重型及危重型患者发热、咳痰、头痛头晕比例高于轻型(P < 0.05)。重型及危重型患者血小板降低,谷草转氨酶、肌酐、肌红蛋白、肌钙蛋白升高(P < 0.05)。影像学表现为单侧或双侧的多发斑片状影或磨玻璃影。重型及危重型患者应用抗病毒药物的数量、抗菌药物、糖皮质激素、丙种球蛋白、胸腺法新及氧疗法的比例更高(P < 0.05)。 结论既往合并多种基础疾病、高龄、肥胖的患者更易进展为重型或危重型, 且可能导致多个系统或器官的异常。 Abstract:ObjectiveTo analyze the epidemiology and clinical characteristics of patients with COVID-19, and provide reference for prevention and control strategies. MethodsWe selected 41 patients with COVID-19 of Linyi, including 9 patients with mild symptom, 25 patients with general symptom, 7 patients with severe and critical symptom. The epidemiological and clinical characteristics were analyzed. ResultsThe median age was 41 years old (31.6-53.6 years old), the male to female ratio 1.56:1, median BMI 25.1 kg/m2(22.4-27.6 kg/m2). The severe and critical patients were older, more obese, and had more underlying diseases. Fifteen cases(36.6%) were clearly related to Hubei, 33 clustered cases (80.5%), the median incubation period 6.5 d (4.0-11.5 d).The main first symptoms of the patients included fever (65.9%), dry cough (78.0%), the proportion of fever, sputum, and headache. The dizziness in severe and critical patients was significantly higher than that in mild group. For severe and critical patients, PLT was significantly reduced. AST, Cr, Myo, and cTnI were significantly increased. CT images showed multiple patchy shadows or ground glass shadows on one or both sides. The number of antiviral drugs, antibiotics, glucocorticoids, gamma globulin, thymus fascin and oxygen therapy were higher in severe and critical patients. ConclusionsPatients with multiple underlying diseases, advanced age, and obesity are more likely to develop into severe or critical. It causes abnormalities in multiple systems or organs. -
Key words:
- COVID-19 /
- pneumonia /
- epidemiology /
- clinical characteristic
-
表 1 COVID-19患者流行病学与临床特征比较
Table 1. The epidemiological and clinical characteristics of COVID-19 [n(%)]
Index All patients
(n=41)Mild group
(n=9)General group
(n=25)Severe or critical group
(n=7)P Age (years)* 41.0 (31.6-53.6) 25.5 (16.4-33.6) 41.38 (35.8-51.9) 62.97 (47.5-75.5) < 0.001 ≤18 2(4.9) 2 (22.2) 0 (0) 0 (0) 0.006 19-40 17(41.5) 6 (66.7) 10(40.0) 1 (14.3) 41-65 19(46.3) 1 (11.1) 14(56.0) 4 (57.1) ≥66 3(7.3) 0 (0) 1(4.0) 2 (28.6) Sex 0.241 Male 25(61.0) 4 (44.4) 15(60.0) 6 (85.7) Female 16(39.0) 5 (55.6) 10(40.0) 1 (14.3) BMI (kg/m2)* 25.1 (22.4-27.6) 22.9 (19.1-23.8) 26.1 (23.3-27.7) 24.6 (22.0-29.1) 0.007 < 24 15(37.5) 7 (77.8) 6 (24.0) 2 (28.6) 0.016 ≥24 26(63.4) 2 (22.2) 19(76.0) 5 (71.4) Comorbidities 0.007 No comorbidities 25(61.0) 9 (100.0) 15(60.0) 1 (14.3) 1 comorbidity 7 (17.1) 0 (0) 6 (24.0) 1 (14.3) 2 comorbidities 6 (14.6) 0 (0) 3 (12.0) 3 (42.9) 3 comorbidities 3(7.3) 0 (0) 1(4.0) 2 (28.6) Types of comorbidities Hypertension 7 (17.1) 0 (0) 4 (16.0) 3 (42.9) 0.076 Diabetes 5 (12.2) 0 (0) 3 (12.0) 2 (28.6) 0.223 Respiratory disease 2(4.9) 0 (0) 2(8.0) 0 (0) 0.510 Endocrine disease 2(4.9) 0 (0) 2(8.0) 0 (0) 0.510 Kindey disease 3(7.3) 0 (0) 0 (0) 3 (42.9) < 0.001 Liver disease 1(2.4) 0 (0) 1(4.0) 0 (0) 0.720 Digestive disease 2(4.9) 0 (0) 1(4.0) 1 (14.3) 0.399 Connective disease 1(2.4) 0 (0) 0 (0) 1 (14.3) 0.083 Exposure to source of transmission within 14 days 0.785 Recently been to Wuhan or other areas of Hubei Province 15(36.6) 4 (44.4) 9 (36.0) 2 (28.6) Contacted with people from Wuhan 15(36.6) 4 (44.4) 8 (32.0) 3 (42.9) Contacted with local confirmed patients 11(26.8) 1 (11.1) 8 (32.0) 2 (28.6) Incubation period (days)* 6.5 (4.0-11.5) 13.0 (2.0-16.0) 6.0 (4.0-8.3) 10.0 (3.0-13.0) 0.338 Time from illness onset to first hospital admission (days)* 2 (1.0-5.5) 2 (1.0-2.0) 4 (1.0-7.5) 1 (0-4.0) 0.151 Clustered onset 33(80.5) 8 (88.9) 19(76.0) 6 (85.7) 0.655 Symptoms Fever≥37.3 ℃ 27(65.9) 0 (0.0) 20(80.0) 7 (100.0) < 0.001 Dry cough 32(78.0) 5 (55.6) 20(80.0) 7 (100) 0.096 Expectoration 9 (22.0) 0 (0) 5 (20.0) 4 (57.1) 0.022 Snivel 4(9.8) 0 (0) 3 (12.0) 1 (14.3) 0.528 Chill 5 (12.2) 0 (0) 4 (16.0) 1 (14.3) 0.446 Fatigue, muscle aches 4(9.8) 0 (0) 3 (12.0) 1 (14.3) 0.528 Headache, dizziness 6 (14.6) 0 (0) 2(8.0) 4 (57.1) 0.002 Dyspnea 10(24.4) 0 (0) 8 (32.0) 2 (28.6) 0.153 Diarrhea 3(7.3) 0 (0) 2(8.0) 1 (14.3) 0.541 *以M (P25, P75)表示. 表 2 COVID-19患者实验室检查与影像学检查数据
Table 2. Laboratory and chest radiography findings of COVID-19 patients [n(%)]
Index All patients
(n=41)Mild group
(n=9)General group
(n=25)Severe or critical group
(n=7)P White blood cell count
(×109/L, 3.5-9.5)*5.3 (4.2-7.5) 5.3 (4.9-8.6) 5.9 (4.2-8.0) 4.8 (3.5-6.4) 0.519 < 4 10(24.4) 1 (11.1) 6 (24.0) 3 (42.9) 0.521 4-10 24(58.5) 6 (66.7) 14(56.0) 4 (57.1) > 10 7 (17.1) 2 (22.2) 5 (20.0) 0 (0.0) Neutrophil count
(×109/L, 1.8-6.3)*3.3 (2.0-5.5) 3.2 (2.3-6.6) 3.6(2.0-5.8) 2.7 (1.7-4.4) 0.679 Lymphocyte count
(×109/L, 1.1-3.2)*1.2 (0.8-2.0) 1.8 (1.2-2.1) 1.1 (0.8-2.0) 1.2 (0.6-1.8) 0.331 < 1.0 14(34.1) 1 (11.1) 10(40.0) 3 (42.9) 0.254 ≥1.0 27(65.9) 8 (88.9) 15 (60.0) 4 (57.1) Platelet count
(×109/L, 125-350)*238.0 (192.5-312.5) 283.0(241.0-331.0) 238.0 (204.5-332.5) 192.0 (148.0-195.0) 0.024 D-dimer (μg/mL,0-0.5)* 0.4 (0.2-0.7) 0.8 (0.4-1.1) 0.4 (0.2-0.6) 0.4 (0.3-5.4) 0.139 < 0.5 23(62.2) 3 (37.5) 15(65.2) 5 (83.3) 0.192 ≥0.5 14(37.8) 5 (62.5) 8 (34.8) 1 (16.7) Alanine aminotransferase
(U/L, 9-50)*20.6 (17.1-31.5) 17.0 (15.0-19.2) 25.1 (18.0-46.5) 22.0 (17.0-36.0) 0.055 Aspartate aminotransferase
(U/L, 15-40)*22.0 (17.7-30.0) 18.0 (14.0-20.4) 25.1 (18.0-30.8) 22.0 (16.0-25.9) 0.042 < 40 34(89.5) 7 (100.0) 21(87.5) 6 (85.7) 0.598 ≥40 4 (10.5) 0 (0) 3 (12.5) 1 (14.3) Potassium (mmol/L,3.5-5.3)* 3.8 (3.6-4.0) 3.7 (3.5-3.9) 3.7 (3.6-4.4) 3.9 (3.8-4.2) 0.307 Sodium (mmol/L,137-147)* 135 (133-137) 137.5 (134.0-140.5) 134.2 (132.6-136.0) 137.0 (134.7-140.0) 0.041 Creatine (μmol/L,62-115)* 63.2 (53.4-71.4) 47.2 (45.2-63.9) 64.4 (55.3-70.1) 62.3 (59.6-78.9) 0.038 ≤133 37(97.4) 8 (100.0) 22 (95.7) 7 (100.0) 0.715 > 133 1(2.6) 0 (0) 1 (4.3) 0 (0.0) Creatine kinase (U/L, 38-174) ≤174 23(92.0) 2 (100.0) 15(88.2) 6 (100.0) 0.600 > 174 2(8.0) 0 (0) 2 (11.8) 0 (0) Lactate dehydrogenase (U/L, 109-245) ≤245 17(65.4) 1 (50.0) 11(64.7) 5 (71.4) 0.850 > 245 9 (34.6) 1 (50.0) 6 (35.3) 2 (28.6) Myoglobin (ng/mL, 28-72) ≤72 31(86.1) 7 (100.0) 20(90.9) 4 (57.1) 0.039 > 72 5 (13.9) 0 (0) 2(9.1) 3 (42.9) Troponin T (ng/mL, 0-0.014) ≤0.014 31(88.6) 7 (100.0) 21(95.5) 3 (50.0) 0.005 > 0.014 4 (11.4) 0 (0) 1(4.5) 3 (50.0) Procalcitonin (ng/mL, 0-0.05) 0.04 (0.02-0.06) 0.03 (0.02-0.04) 0.04 (0.02-0.05) 0.06 (0.03-0.09) 0.075 < 0.1 34(89.5) 8 (100.0) 20(87.0) 6 (85.7) 0.549 ≥0.1 4 (10.5) 0 (0) 3 (13.0) 1 (14.3) ESR > 15 mm/h 23/34 (67.6) 5/7 (71.4) 15/20 (75.0) 3/7 (42.9) 0.286 CRP > 10 mg/L 23/41 (56.1) 2/9 (22.2) 16/25 (64.0) 5/7 (71.4) 0.064 Chest computed tomograms 0.014 Bilateral involvement 32(78.0) 4 (44.4) 21(84.0) 7 (100.0) Unilateral involvement 4(9.8) 1 (11.1) 3 (12.0) 0 (0) No involvement 5 (12.2) 4 (44.4) 1(4.0) 0 (0.0) *以M (P25, P75)表示. 表 3 COVID-19患者治疗手段与结局
Table 3. Treatments and outcomes of COVID-19 patients [n(%)]
Treatments and outcomes All patients (n=41) Mild group (n=9) General group (n=25) Severe or critical group (n=7) P Chinese medicine 38(92.7) 7 (77.8) 24(96.0) 7 (100.0) 0.142 Antiviral therapy 0.052 No antiviral therapy 1(2.4) 1 (11.1) 0 (0) 0 (0) 1 antiviral therapy 1(2.4) 0 (0) 1(4.0) 0 (0) 2 antiviral therapy 21(51.2) 3 (33.3) 17(68.0) 1 (14.3) ≥3 antiviral therapy 18(43.9) 5 (55.6) 7 (28.0) 6 (85.7) Interferon alpha inhalation 40(97.6) 8 (88.9) 25 (100.0) 7 (100.0) 0.162 Lopinavir/ritonavir 39(95.1) 8 (88.9) 24(96.0) 7 (100.0) 0.562 Arbidol 11(26.8) 5 (55.6) 3 (12.0) 3 (42.9) 0.024 Ribavirin 5 (12.2) 0 (0) 1(4.0) 4 (57.1) < 0.001 Other treatments Antibotic 21(51.2) 0 (0) 14(56.0) 7 (100.0) < 0.001 Glucocorticoid 16(39.0) 0 (0) 10(40.0) 6 (85.7) 0.002 Immunoglobulin 12(29.3) 0 (0) 7 (28.0) 5 (71.4) 0.008 Thymalfasin 20(48.8) 1 (11.1) 12(48.0) 7 (100.0) 0.002 Oxygen therapy Intranasal oxygen inhalation 18(43.9) 0 (0) 12(48.0) 6 (85.7) 0.002 Mask oxygen 4(9.8) 0 (0) 0 (0) 4 (57.1) < 0.001 High low oxygen 7 (17.1) 0 (0) 1(4.0) 6 (85.7) < 0.001 Non-invasive mechanical ventilation 2(4.9) 0 (0) 0 (0) 2 (28.6) 0.006 ECMO 1(2.4) 0 (0) 0 (0) 1 (14.3) 0.083 Plasmapheresis 1(2.4) 0 (0) 0 (0) 1 (14.3) 0.083 Outcome 0.083 Hospital discharge 40(97.6) 9 (100.0) 25 (100.0) 6 (85.7) ICU admission 1(2.4) 0 (0) 0 (0) 1 (14.3) Death 0 (0) 0 (0) 0 (0) 0 (0) -
[1] Li Q, Guan XH, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia[J]. N Engl J Med, 2020, 382(13): 1199-207. doi: 10.1056/NEJMoa2001316 [2] Jiang SB, Xia S, Ying TL, et al. A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome[J]. Cell Mol Immunol, 2020, 17(5): 554-62. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1097/MAT.0000000000001176 [3] Huang CL, Wang YM, Li XW, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China[J]. Lancet, 2020, 395(10223): 497-506. https://www.sciencedirect.com/science/article/pii/S0140673620301835 [4] Kritas SK, Ronconi G, Caraffa A, et al. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy [J]. J Biol Regul Homeost Agents, 2020, 34(1): 9-14. [5] 中华人民共和国国家卫生健康委员会.新型冠状病毒肺炎诊疗方案(试行第六版).[EB/OL].[2020-2-19].http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml. [6] Guan W, Ni Z, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China[J]. N Engl J Med, 2020, doi: 10.1056/NEJMoa2002032. [7] 杨凯, 肖玲燕, 刘永福, 等. 57例非疫区新型冠状病毒肺炎流行病学及临床特点分析[J].第三军医大学学报, 2020, 42(6): 555-9. [8] 中国疾病预防控制中心新型冠状病毒肺炎应急响应机制流行病学组, 张彦平.新型冠状病毒肺炎流行病学特征分析[J].中华流行病学杂志, 2020, 41(2): 145-6, 147. http://d.old.wanfangdata.com.cn/Periodical/zgrsghbzz202005006 [9] Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series[J]. BMJ, 2020, 368: m606-73. http://d.old.wanfangdata.com.cn/Periodical/zh-bmj-c202004009 [10] Lee N, Hui DSC, Wu AHB, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong[J]. N Engl J Med, 2003, 348 (20): 1986-94. doi: 10.1056-NEJMoa030685/ [11] Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study[J]. Lancet Infect Dis, 2013, 13(9): 752-61. https://www.sciencedirect.com/science/article/pii/S1473309913702044 [12] 房晓伟, 梅清, 杨田军, 等. 2019新型冠状病毒感染的肺炎79例临床特征及治疗分析[J].中国药理学通报, 2020, 36(4): 453-9. http://d.old.wanfangdata.com.cn/Periodical/zgylxtb202004002 [13] 赵双全, 周永生, 殷亮, 等.新型冠状病毒肺炎的临床特征和CT表现[J].分子影像学杂志, 2020, 43(1): 59-63. doi: 10.12122/j.issn.1674-4500.2020.01.13 -







 下载:
下载: