Hyaluronic acid-Gd2O3@MSN, nanoparticle probe of MRI for targeted identification of atherosclerosis
-
摘要:
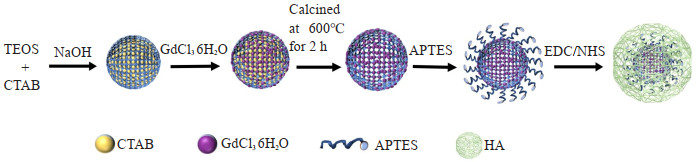
目的 探究合成透明质酸(HA)修饰的介孔二氧化硅MR纳米探针HA-Gd2O3@MSN的性能以及成像特点,为动脉粥样硬化疾病提供新的检查技术手段。 方法 以CTAB为模板,正硅酸四乙酯和十六烷基三甲基溴化铵在碱性条件下制备成MSN,滴加GdCl36H2O变成Gd2O3@MSN,在600℃下煅烧2 h去除十六烷基三甲基溴化铵模板,最后制备成分子探针Gd2O3@MSN,再通过酰胺缩合制备成HA-Gd2O3@MSN。运用透射电镜观察其形貌特征,动态光散射法检测其水合粒径和Zeta电位。运用3.0T MR观察其成像效果,并利用ICP-MS数据分析探针的弛豫率。 结果 合成的探针水动力尺寸为223.5±10.5 nm,Zeta电位为-13.04 mV,弛豫率为11.023 mmol· L-1· s-1);随着分子探针浓度逐渐升高,T1信号也随之增强。体外细胞试验研究显示透明质酸-Gd2O3@MSN以HA依赖的方式靶向巨噬细胞表面受体CD44。在细胞毒性试验中发现HA包被的纳米探针毒性较小。 结论 HA修饰的介孔二氧化硅MR纳米探针HA-Gd2O3@MSN T1弛豫率高,细胞毒性小,靶向效果好,具有较好的MR成像增强效果,为进一步早期识别动脉粥样硬化斑块奠定基础。 Abstract:Objective To synthesize a hyaluronic acid (HA)-modified mesoporous silica nanoprobe (MSN) for MRI, which is HAGd2O3@MSN. Its performance and imaging characteristics were preliminarily studied, so as to provide a new technical tool for the diagnosis of atherosclerosis. Methods The cetyltrimethylammonium bromide (CTAB) was utilized as the template, and tetraethyl orthosilicate and CTAB were formulated into MSN under alkaline conditions. GdCl36H2O was incrementally introduced to produce the Gd2O3@MSN. Subsequently, the CTAB template was eliminated through calcination at 600 ℃ for 2 h, which led to the formation of the molecular probe Gd2O3@MSN. The HA-Gd2O3@MSN was then prepared through amide condensation. The morphology of HA-Gd2O3@MSN was examined by transmission electron microscopy, while its hydrodynamic diameter and zeta potential were measured by dynamic light scattering technique. The imaging results were observed by a 3.0T MR scanner and the relaxation rate of the molecular probe Gd2O3@MSN was analyzed using data from the inductively coupled plasma mass spectrometry testing. Results The hydrodynamic diameter of the synthesized probe HA-Gd2O3@MSN was 223.5±10.5 nm, the zeta potential of HA-Gd2O3@MSN was -13.04 mV, with its relaxation rate being 11.023 mmol· L-1· s-1. As the concentration of the molecular probe increased incrementally, the T1 signal was correspondingly enhanced. Through in vitro cellular studies, we demonstrated that HA-Gd2O3@MSN was capable to bind and target the CD44 receptors on the surface of macrophages in a HA-dependent manner. Furthermore, the HA-coated nanoprobes were found to be less toxic in cytotoxicity assays. Conclusion The nanoprobe HA-Gd2O3@MSN, with its high T1 relaxation rate, low cytotoxicity, excellent targeting capacity and good MR imaging enhancement effects, it lays the foundation for identification of atherosclerotic plaques at earlier stages. -
Key words:
- hyaluronic acid /
- mesoporous silica /
- MR molecular probes /
- nanoparticle probe
-
图 4 纳米探针的高角环形暗场扫描透射及元素mapping分析
Figure 4. High-angle annular dark-field scanning transmission and elemental mapping analysis of nanoprobes.
A: STEM-HAADF analysis of HA-Gd2O3@MSN; B: Mapping analysis of element Gd; C: Mapping analysis of element Si; D: Mapping analysis of element O; E: Merged image of mapping analysis of elements Gd, Si and O.
-
[1] Htun NM, Chen YC, Lim B, et al. Near-infrared autofluorescence induced by intraplaque hemorrhage and heme degradation as marker for high-risk atherosclerotic plaques[J]. Nat Commun, 2017, 8: 75. doi: 10.1038/s41467-017-00138-x [2] Zhang J, Li C, Zhang X, et al. In vivo tumor-targeted dual-modal fluorescence/CT imaging using a nanoprobe co-loaded with an aggregation-induced emission dye and gold nanoparticles[J]. Biomaterials, 2015, 42: 103-11. doi: 10.1016/j.biomaterials.2014.11.053 [3] Lairez O, Fayad ZA. Imaging of atherosclerosis: can molecular imaging do more?[J]. Arch Cardiovasc Dis, 2013, 106(11): 551-3. doi: 10.1016/j.acvd.2013.06.001 [4] Wolf KJ, Kumar S. Hyaluronic acid: incorporating the bio into the material[J]. ACS Biomater Sci Eng, 2019, 5(8): 3753-65. doi: 10.1021/acsbiomaterials.8b01268 [5] Goh EJ, Kim KS, Kim YR, et al. Bioimaging of hyaluronic acid derivatives using nanosized carbon dots[J]. Biomacromolecules, 2012, 13(8): 2554-61. doi: 10.1021/bm300796q [6] Lee MY, Yang JA, Jung HS, et al. Hyaluronic acid-gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection[J]. ACS Nano, 2012, 6(11): 9522-31. doi: 10.1021/nn302538y [7] Hossaini NS, Rashidijahanabad Z, Ramadan S, et al. Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles[J]. Nanoscale, 2020, 12(17): 9541-56. doi: 10.1039/D0NR00308E [8] Hou XY, Lin H, Zhou XD, et al. Novel dual ROS-sensitive and CD44 receptor targeting nanomicelles based on oligomeric hyaluronic acid for the efficient therapy of atherosclerosis[J]. Carbohydr Polym, 2020, 232: 115787. doi: 10.1016/j.carbpol.2019.115787 [9] 梁治平, 曾旭文. Gd(Ⅲ)类分子探针在MRI分子影像学中的研究进展[J]. 临床放射学杂志, 2006, 25(8): 782-4. doi: 10.3969/j.issn.1001-9324.2006.08.022 [10] Kilpelainen M, Riikonen J, Vlasova MA, et al. In vivo delivery of a peptide, ghrelin antagonist, with mesoporous silicon microparticles[J]. J Control Release, 2009, 137(2): 166-70. doi: 10.1016/j.jconrel.2009.03.017 [11] Yang PP, Gai SL, Lin J. Functionalized mesoporous silica materials for controlled drug delivery[J]. Chem Soc Rev, 2012, 41(9): 3679- 98. doi: 10.1039/c2cs15308d [12] 朱飞鹏. 基于介孔二氧化硅双模态多功能探针的制备及实验研究[D]. 广州: 南方医科大学, 2013. [13] He KW, Li JJ, Shen YX, et al. pH-Responsive polyelectrolyte coated gadolinium oxide-doped mesoporous silica nanoparticles (Gd2O3@MSNs) for synergistic drug delivery and magnetic resonance imaging enhancement[J]. J Mater Chem B, 2019, 7(43): 6840-54. doi: 10.1039/C9TB01654F [14] Shatadal G, Sayanta D, Abhijit S, et al. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency[J]. Colloids Surf B Biointerfaces, 2021, 197: 111404. doi: 10.1016/j.colsurfb.2020.111404 [15] Dweck MR, Aikawa E, Newby DE, et al. Noninvasive molecular imaging of disease activity in atherosclerosis[J]. Circ Res, 2016, 119(2): 330-40. doi: 10.1161/CIRCRESAHA.116.307971 [16] Yamazaki T, Nohara R, Daida H, et al. Intensive lipid-lowering therapy for slowing progression as well as inducing regression of atherosclerosis in Japanese patients[J]. Int Heart J, 2013, 54(1): 33- 9. doi: 10.1536/ihj.54.33 [17] 田泾, 高申. 基于疾病微环境靶向的精准纳米靶向技术研究[J]. 医学研究生学报, 2019, 32(5): 479-83. doi: 10.16571/j.cnki.1008-8199.2019.05.006 [18] 谷弘谦, 陈颂, 尹强强, 等. 以Cy5.5为包裹的团簇磁共振分子探针G4-Gd2O3-Cy5.5的构建与表征[J]. 临床放射学杂志, 2021, 40(10): 2016-20. https://www.cnki.com.cn/Article/CJFDTOTAL-LCFS202110036.htm [19] Li ZT, Guo J, Zhang MM, et al. Gadolinium-coated mesoporous silica nanoparticle for magnetic resonance imaging[J]. Front Chem, 2022, 10: 837032. doi: 10.3389/fchem.2022.837032 [20] 李尚基. 基于氨基化介孔二氧化硅/生物大分子药物控释系统的构建[D]. 常州: 常州大学, 2021. [21] 孙凯. 基于介孔二氧化硅靶向药物递送系统的构建及其抗肿瘤活性的研究[D]. 南京: 东南大学, 2019. [22] Song KC, Tang Z, Song ZL, et al. Hyaluronic acid-functionalized mesoporous silica nanoparticles loading simvastatin for targeted therapy of atherosclerosis[J]. Pharmaceutics, 2022, 14(6): 1265. doi: 10.3390/pharmaceutics14061265 [23] 李忠涛, 陈颂, 郝利国, 等. 以羧基化石墨烯量子点为载体的荧光-磁共振双模态分子探针的制备及体外研究[J]. 中国医疗设备, 2022, 37 (5): 64-8. https://www.cnki.com.cn/Article/CJFDTOTAL-YLSX202205014.htm [24] Ahmad MW, Xu WL, Kim SJ, et al. Potential dual imaging nanoparticle: Gd2O3 nanoparticle[J]. Sci Rep, 2015, 5: 8549. doi: 10.1038/srep08549 [25] 张亚力. 基于整合素αvβ3的磁共振/荧光双模态分子探针cRGD-GdCy5.5的制备及其靶向性研究[D]. 南宁: 广西医科大学, 2019. [26] Liu S, Zhao Y, Shen ML, et al. Hyaluronic acid targeted and pH-responsive multifunctional nanoparticles for chemo-photothermal synergistic therapy of atherosclerosis[J]. J Mater Chem B, 2022, 10 (4): 562-70. doi: 10.1039/D1TB02000E -







 下载:
下载: