Mechanism of Chaihu Shugan powder in improving depression rats with liver-qi stagnation based on microPET/CT
-
摘要:
目的 观察柴胡疏肝散对肝气郁结型抑郁症大鼠行为改善,并探索其临床疗效机制是否是通过改善脑葡萄糖代谢发挥抗抑郁作用。 方法 雄性Wistar大鼠40只,按体质量匹配原则随机分为对照组、模型组和柴胡疏肝散高、低剂量组,10只/组,运用慢性束缚应激结合孤养法建立肝气郁结型大鼠模型,柴胡疏肝散高、低剂量组分别用1.04 g/mL及0.52 g/mL的浓缩药液灌胃,对照组与模型组用生理盐水灌胃,灌胃从实验开始之日起,1次/d,持续21 d。观察旷场实验和糖水偏好实验变化,使用小型正电子发射断层显像与CT摄影融合术观察大鼠感兴趣脑区的脑葡萄糖代谢变化。 结果 行为学提示:与对照组相比,模型组大鼠糖水偏好程度下降(P < 0.05),旷场实验不动时间延长(P < 0.05)。脑代谢提示:模型组代谢增高脑区:双侧延髓、左侧小脑(P < 0.05),代谢减低脑区:双侧胼胝体、双侧M1、双侧M2、左侧纹状体、右侧丘脑、右侧海马、右侧前额叶皮质、右侧扣带皮质、右侧S1、右侧S2、右侧岛叶皮质(P < 0.05)。与模型组相比,高、低剂量组糖水偏好程度增高(P < 0.05),旷场实验不动时间减少(P < 0.05);低剂量组代谢增高脑区:双侧纹状体、双侧海马、双侧胼胝体、双侧前额叶皮质、左侧下丘、左侧M2、右侧听觉皮质、右侧丘脑、右侧M1、右侧S1、右侧S2、右侧岛叶皮质(P < 0.05),代谢减低脑区:左侧延髓、左侧视觉皮质、左侧小脑(P < 0.05);高剂量组代谢增高脑区:右侧前额叶皮质(P < 0.05);其它感兴趣脑区二者的差异无统计学意义(P>0.05)。 结论 肝气郁结证发病机制为慢性应激结合孤养法降低情绪认知和行为调节相关脑区葡萄糖代谢,使之失代偿,同时使左脑纹状体、小脑脑区发生葡萄糖摄取竞争,柴胡疏肝散可改善肝气郁结型抑郁症大鼠行为,其主要通过改善与情绪认知、行为等相关脑区及改善左脑纹状体、小脑脑区葡萄糖摄取竞争从而发挥抗抑郁效应。 -
关键词:
- 抑郁症 /
- 肝气郁结证 /
- 柴胡疏肝散 /
- microPET/CT
Abstract:Objective To investigate the effects of Chaihu Shugan powder on the behavior of rats with liver-qi stagnation depression and to explore the potential antidepressant mechanism of Chaihu Shugan powder through the improvement of brain glucose metabolism. Methods Forty male Wistar rats were randomly divided into control group, model group, low-dose Chaihu Shugan powder group, and high-dose Chaihu Shugan powder group, with 10 rats per group. A rat model of liver-qi stagnation was established by using chronic restraint stress combined with solitary rearing. The high and low dose Chaihu Shugan powder groups were administered 1.04 g/mL and 0.52 g/mL concentrated solutions by gavage, respectively. The control and model groups were treated with normal saline by gavage once daily for 21 days from the beginning of the experiment. Results Behavioral indicators: Compared with the control group, the sucrose preference of the rats in the model group significantly decreased (P < 0.05), and the immobility time in the open field test significantly increased (P < 0.05). Brain metabolism indicators: The brain regions with increased metabolism in the model group were the bilateral medulla oblongata and left cerebellum (P < 0.05), while the brain regions with decreased metabolism included bilateral corpus callosum, bilateral M1, bilateral M2, left striatum, right thalamus, right hippocampus, right prefrontal cortex, right cingulate cortex, right S1, right S2, and right insular cortex (P < 0.05). In comparison with the model group, both high-and low-dose groups showed a significant increase in sucrose preference (P < 0.05) and a significant decrease in immobility time in the open field test (P < 0.05). In the low-dose group, the brain regions with increased metabolism encompassed bilateral striatum, bilateral hippocampus, bilateral corpus callosum, bilateral prefrontal cortex, left inferior colliculus, left M2, right auditory cortex, right thalamus, right M1, right S1, right S2, right insular cortex (P < 0.05), whereas the brain regions with decreased metabolism included left medulla, left visual cortex, and left cerebellum (P < 0.05). The high-dose group displayed increased metabolism in the right prefrontal cortex (P < 0.05). No significant differences were observed in other regions of interest (P>0.05). Conclusion The pathogenesis of liver-qi stagnation syndrome may involve chronic stress combined with isolation, resulting in decreased glucose metabolism in brain regions related to emotional cognition and behavioral regulation, leading to decompensation and concurrent glucose uptake competition in the left striatum and cerebellar brain regions. Chaihu Shugan powder can improve the behavior of rats with liver-qi stagnation depression by exerting its antidepressant effect primarily through enhancement of emotional cognition and behavioral-related brain regions, and ameliorating glucose uptake competition in the left striatum and cerebellar brain regions. -
Key words:
- depression /
- liver-qi stagnation /
- Chaihu Shugan powder /
- microPET/CT
-
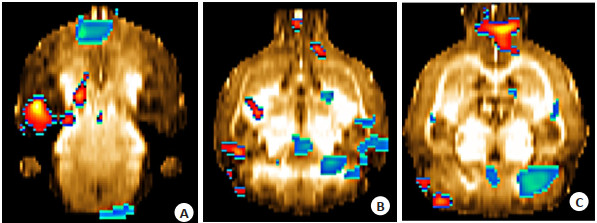
图 1 与对照组相比,模型组脑葡萄糖代谢增高、减低脑区
Figure 1. Compared with the control group, brain regions glucose metabolism increased and decreased in the model group.
A: Hippocampus, corpus callosum; B: Prefrontal cortex, thalamus, inferior colliculus, M1, M2, S1, S2, cingulated cortex, corpus striatum, insular cortex, auditory cortex; C: Cerebellum, medulla oblongata, visual cortex. The red-yellow colors are the regions with increased metabolism, and the blue-green colors are the regions with decreased metabolism, and the regions with decreased metabolism and increased metabolism have overlapping colors.
图 2 与模型组相比,低剂量组脑葡萄糖代谢增高、减低脑区
Figure 2. Compared with the model group, brain regions glucose metabolism increased and decreased in the low-dose group.
A: Hippocampus, corpus callosum; B: Prefrontal cortex, thalamus, inferior colliculus, M1, M2, S1, S2, cingulated cortex, corpus striatum, insular cortex, auditory cortex; C: Cerebellum, medulla oblongata, visual cortex. The red-yellow colors are the regions with increased metabolism, and the blue-green colors are the regions with decreased metabolism, and the regions with decreased metabolism and increased metabolism have overlapping colors.
图 3 与模型组相比,高剂量组脑葡萄糖代谢增高、减低脑区
Figure 3. Comparison with model group, brain regions glucose metabolism increased and decreased in the high- dose group.
A: Hippocampus, corpus callosum; B: Prefrontal cortex, thalamus, inferior colliculus, M1, M2, S1, S2, cingulated cortex, corpus striatum, insular cortex, auditory cortex; C: Cerebellum, medulla oblongata, visual cortex. The red-yellow colors are the regions with increased metabolism, and the blue-green colors are the regions with decreased metabolism, and the regions with decreased metabolism and increased metabolism have overlapping colors.
图 4 与低剂量组相比,高剂量组脑葡萄糖代谢增高、减低脑区
Figure 4. Comparison with the low-dose group, brain regions glucose metabolism increased and decreased in the high-dose group.
A: Hippocampus, corpus callosum; B: Prefrontal cortex, thalamu, inferior colliculus, M1, M2, S1, S2, cingulated cortex, corpus striatum, insular cortex, auditory cortex; C: Cerebellum, medulla oblongata, visual cortex. The red-yellow colors are the regions with increased metabolism, and the blue- green colors are the regions with decreased metabolism, and the regions with decreased metabolism and increased metabolism have overlapping colors.
表 1 各组蔗糖水实验变化比较
Table 1. Comparison of sucrose water experiment in each group (%, n=10, Mean±SD
Group Degree of sucrose water preference before intervention Degree of sucrose water preference after 3 weeks of intervention Control group 85.0±0.82 86.9±0.54 Model group 85.5±0.41 70.4±0.35* Low-dose group 86.4±0.90 82.8±0.99▲ High-dose group 85.5±0.58 81.6±0.84▲ *P < 0.05 vs control group; ▲P < 0.05 vs model group. 表 2 各组开野实验变化比较
Table 2. Comparison of open-field experiment changes in each group (n=10, Mean±SD
Group Times of horizontal movements Times of vertical movements Length of stay in the central area(s) Number of fecal pellets Control group 55.52±14.66 16.04±4.66 1.25±2.03 1.32±1.24 Model group 26.90±15.57* 8.52±2.80* 3.17±2.65 7.06±3.66* Low-dose group 46.04±12.33▲ 14.87±5.52▲ 2.81±2.14 1.25±1.01▲ High-dose group 47.15±13.80▲ 11.95±3.09▲ 1.67±1.89 2.34±1.17▲ *P < 0.05 vs control group; ▲P < 0.05 vs model group. 表 3 模型组与对照组不同脑区相对葡萄糖代谢率变化比较
Table 3. Comparison of relative glucose metabolic rate in different brain regions between model group and control group (n=10, Mean±SD)
Brain region Control group Model group Left corpus striatum 1.07486±0.02460 1.02725±0.03359* Left medulla oblongata 0.93674±0.08531 1.01784±0.08111* Left cerebellum 1.04118±0.09803 1.11027±0.03221* Left corpus callosum 1.03780±0.05123 0.99216±0.02931* Left M1 0.99808±0.07689 0.93636±0.04580* Left M2 1.06499±0.10819 0.97459±0.04030* Right thalamus 1.14833±0.04277 1.11027±0.03693* Right medulla oblongata 1.00976±0.07858 1.09288±0.09819* Right hippocampus 1.04963±0.03839 1.01059±0.02546* Right corpus callosum 1.04392±0.04199 1.00301±0.02586* Right prefrontal cortex 1.14400±0.04386 1.08218±0.02593* Right cingulated cortex 1.09671±0.10480 1.01469±0.03777* Right M1 1.02810±0.09376 0.94466±0.03318* Right M2 1.07593±0.11416 0.98556±0.03734* Right S1 1.00563±0.04336 0.95574±0.02484* Right S2 1.01604±0.03697 0.96487±0.02686* Right insular cortex 0.98743±0.07179 0.92576±0.03311* *P < 0.05 vs control group. This experiment obtained a total of 44 sets of data. Listing all the data would take up a huge amount of space, so only the data with statistical significance is listed. 表 4 低剂量组、高剂量组与模型组不同脑区相对葡萄糖代谢率变化比较
Table 4. Comparison of relative glucose metabolic rate in different brain regions between low-dose group, high-dose group and model group (n=10, Mean±SD)
Brain region Model group Low-dose group High-dose group Left corpus striatum 1.02725±0.03359 1.06729±0.02972▲ 1.05616±0.06592 Left medulla oblongata 1.01784±0.08111 0.95690±0.02402▲ 1.00923±0.07525 Left inferior colliculus 1.16477±0.04259 1.22799±0.06413▲ 1.21003±0.06411 Left visual cortex 0.85974±0.05589 0.79350±0.06542▲ 0.89999±0.09641* Left cerebellum 1.11027±0.03221 1.04803±0.03947▲ 1.06086±0.09602 Left hippocampus 1.02593±0.03364 1.06242±0.02335▲ 1.03241±0.04048 Left corpus callosum 0.99216±0.02931 1.01954±0.02372▲ 0.99209±0.07030 Left prefrontal cortex 1.10101±0.05407 1.17155±0.06835▲ 1.15035±0.07756 Left M2 0.97459±0.04030 1.01667±0.03803▲ 1.01479±0.10404 Right corpus striatum 1.04484±0.02560 1.07362±0.02495▲ 1.05920±0.05883 Right thalamus 1.11027±0.03693 1.14687±0.01397▲ 1.11287±0.06319 Right auditory cortex 0.96606±0.03982 1.01910±0.03736▲ 0.99829±0.04764 Right hippocampus 1.01059±0.02546 1.06271±0.02824▲ 1.03129±0.03607* Right corpus callosum 1.00301±0.02586 1.03226±0.02503▲ 1.01300±0.07025 Right prefrontal cortex 1.08218±0.02593 1.15355±0.05609▲ 1.14995±0.08731▲ Right M1 0.94466±0.03318 0.98005±0.03661▲ 0.97561±0.09001 Right S1 0.95574±0.02484 0.98478±0.02877▲ 0.96434±0.05408 Right S2 0.96487±0.02686 0.99711±0.02877▲ 0.97511±0.05418 Right insular cortex 0.92576±0.03311 0.98170±0.04974▲ 0.96472±0.05944 ▲P < 0.05 vs model group; *P < 0.05 vs low-dose group -
[1] 沈宛颖, 曾昱兴, 李文豪, 等. 基于GBD大数据的中国抑郁负担现状和趋势分析[J]. 职业与健康, 2021, 37(8): 1087-92. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYJK202108021.htm [2] 潘瑾, 王墉, 高志礼, 等. 中医经典名方治疗抑郁症的研究进展[J]. 世界科学技术-中医药现代化, 2022, 24(7): 2809-16. https://www.cnki.com.cn/Article/CJFDTOTAL-SJKX202207033.htm [3] 邹东凌, 傅晓芸, 黄淑玲. 黄淑玲治疗围绝经期汗证的临床经验探析[J]. 广州中医药大学学报, 2023, 40(4): 1002-7. https://www.cnki.com.cn/Article/CJFDTOTAL-REST202304032.htm [4] 王英娜, 高天舒, 李品, 等. 柴胡疏肝散化裁方治疗肝郁脾虚证桥本甲状腺炎合并甲减患者临床疗效观察[J]. 浙江中医药大学学报, 2023, 47(3): 291-5. https://www.cnki.com.cn/Article/CJFDTOTAL-BHON202303013.htm [5] 于洋, 刘凯莉, 李昱帅, 等. 柴胡疏肝散联合俞募配穴法治疗围绝经期抑郁症[J]. 中医药信息, 2023, 40(4): 57-62. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXN202304009.htm [6] 赵静, 龙彬, 朱丽萍. 柴胡疏肝散在抑郁症治疗中的研究进展[J]. 精神医学杂志, 2018, 31(3): 237-40. doi: 10.3969/j.issn.2095-9346.2018.03.021 [7] 王常瞵, 高冬梅, 高明周, 等. 柴胡疏肝散化学成分和药理作用研究进展及质量标志物的预测分析[J]. 中华中医药学刊, 2022, 40(11): 124- 31, 271. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYHS202211029.htm [8] 侯丽恺, 陈丽, 张乙小, 等. 基于文献计量学的中医脑病证候影像学研究现状分析[J]. 国际医学放射学杂志, 2020, 43(1): 21-5. https://www.cnki.com.cn/Article/CJFDTOTAL-GWLC202001006.htm [9] 刘子旺, 赵海滨, 张秀静, 等. 电针肝俞、期门对肝气郁结模型大鼠正电子发射脑功能成像研究[J]. 北京中医药大学学报, 2012, 35(5): 333-6, 362. https://www.cnki.com.cn/Article/CJFDTOTAL-JZYB201205010.htm [10] 熊振芳, 朱清静. 柴胡疏肝散对慢性束缚应激性肝郁证大鼠的影响[J]. 中国中西医结合消化杂志, 2004, 12(4): 220-1. doi: 10.3969/j.issn.1671-038X.2004.04.010 [11] 严灿, 徐志伟, 吴丽丽, 等. 建立中医情志致病动物模型的思考[J]. 中国临床康复, 2006(3): 155-7. https://www.cnki.com.cn/Article/CJFDTOTAL-XDKF200603058.htm [12] 徐舒, 陈合兵, 李洪, 等". 肝郁证"大鼠模型的建立及代谢组学的初步研究[J]. 中华中医药杂志, 2009, 24(6): 787-91. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY200906035.htm [13] 吴晓玲, 张贵锋. 柴胡疏肝散对抑郁症大鼠行为学及旷场实验的影响[J]. 中国中医药现代远程教育, 2018, 16(2): 90-2. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZYY201802041.htm [14] Fueger B, Czernin J, Hildebrandt I, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice[J]. J Nucl Med, 2006, 47(6): 999-1006. [15] 姚玉璞, 张立平, 郭蓉娟, 等. 从抑郁症的主要证型探讨"见肝之病, 知肝传脾"[J]. 中华中医药杂志, 2013, 28(4): 1081-3. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201304061.htm [16] 吴骥, 邓玮玮, 范敏, 等. PET及PET/CT对抑郁症的研究现状及进展[J]. 中国医学影像技术, 2019, 35(3): 447-50. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXX201903048.htm [17] Huang X, Lai S, Lu X, et al. Cognitive dysfunction and neurometabolic alternations in major depressive disorder with gastrointestinal symptoms[J]. J Affect Disord, 2023, 322: 180-6. [18] Sun N, Liu M, Liu P, et al. Abnormal cortical-striatal-thalamiccortical circuit centered on the thalamus in MDD patients with somatic symptoms: evidence from the REST-meta-MDD project [J]. J Affect Disord, 2023, 323: 71-84. [19] Hu YQ, Zhao CQ, Zhao HF, et al. Abnormal functional connectivity of the nucleus accumbens subregions mediates the association between anhedonia and major depressive disorder[J]. BMC Psychiatry, 2023, 23(1): 282. [20] Chen FY, Wang LY, Ding ZX. Alteration of whole-brain amplitude of low-frequency fluctuation and degree centrality in patients with mild to moderate depression: a resting-state functional magnetic resonance imaging study[J]. Front Psychiatry, 2022, 13: 1061359. [21] Ming QS, Zhang J, Cheng C, et al. Trait-like white matter abnormalities in current and remitted depression[J]. Psychiatry Res Neuroimaging, 2022, 326: 111544. [22] Shin SJ, Kim A, Han KM, et al. Reduced sulcal depth in central sulcus of major depressive disorder[J]. Exp Neurobiol, 2022, 31 (5): 353-60. [23] 陈雅洁, 李月峰. 不同应激时间对大鼠抑郁样行为及脑内神经炎症的动态变化[J]. 医学研究杂志, 2022, 51(12): 32-7. https://www.cnki.com.cn/Article/CJFDTOTAL-YXYZ202212008.htm [24] 付爱兵, 胡佳佳. 抑郁症患者神经细胞18F-FDG摄取代谢的PET/CT差异分析[J]. 精神医学杂志, 2019, 32(2): 119-23. https://www.cnki.com.cn/Article/CJFDTOTAL-SDJB201902014.htm [25] 周群, 李月峰, 朱彦. PET-CT检测首发单相重度抑郁症患者神经环路的葡萄糖代谢水平[J]. 江苏大学学报: 医学版, 2015, 25(4): 335-38. https://www.cnki.com.cn/Article/CJFDTOTAL-ZJYZ201504014.htm [26] Bruder G, Stewart J, McGrath P. Right brain, left brain in depressive disorders: clinical and theoretical implications of behavioral, electrophysiological and neuroimaging findings[J]. Neurosci Biobehav Rev, 2017, 78: 178-91. [27] Sahib AK, Loureiro JR, Vasavada M, et al. Modulation of the functional connectome in major depressive disorder by ketamine therapy[J]. Psychol Med, 2022, 52(13): 2596-605. [28] 高雪松, 赵静洁. 柴胡疏肝散治疗抑郁症研究进展[J]. 河南中医, 2022, 42(4): 629-33. https://www.cnki.com.cn/Article/CJFDTOTAL-HNZY202204033.htm [29] 滕海英, 肖建平, 付仰志, 等. 柴胡疏肝散对慢性不可预见性温和应激抑郁模型大鼠的抗抑郁作用研究[J]. 福建中医药, 2022, 53(12): 19- 22. https://www.cnki.com.cn/Article/CJFDTOTAL-FJZY202212007.htm [30] 张震, 赵博, 刘炽鉴, 等. 柴胡疏肝散对CUMS大鼠眶额叶皮层的保护作用机制[J]. 中药材, 2021, 44(7): 1713-8. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYCA202107031.htm [31] 周莺, 吴丽丽, 陈煜辉. 柴胡疏肝散及其提取物对慢性应激大鼠下丘脑CRH含量的影响[J]. 临床和实验医学杂志, 2006, 5(7): 914-5. https://www.cnki.com.cn/Article/CJFDTOTAL-SYLC200607037.htm [32] 张付民, 刘俊. 柴胡疏肝散调节大鼠额叶环磷酸腺苷/环磷酸腺苷反应元件结合蛋白/脑源性神经营养因子信号通路发挥抗抑郁作用研究[J]. 安徽医药, 2020, 24(4): 646-50. https://www.cnki.com.cn/Article/CJFDTOTAL-AHYY202004003.htm [33] 高雪松, 李丽, 王安娜, 等. 柴胡疏肝散精简方对抑郁大鼠行为学及中缝核内色氨酸代谢的影响[J]. 实验动物科学, 2023, 40(1): 16-21. https://www.cnki.com.cn/Article/CJFDTOTAL-SYDG202301003.htm [34] 肖迪, 刘俊. 柴胡疏肝散对抑郁症模型大鼠前额叶皮层SIRT1/NF- κB信号通路的作用研究[J]. 湖北中医药大学学报, 2021, 23(2): 9- 14. https://www.cnki.com.cn/Article/CJFDTOTAL-HZXX202102002.htm [35] 赵宾宾, 龙清华, 王平, 等. 柴胡疏肝散对MSG-大鼠-肝再生模型肝脏损伤和抑郁症脑区神经营养因子的保护作用[J]. 时珍国医国药, 2018, 29(12): 2833-7. https://www.cnki.com.cn/Article/CJFDTOTAL-SZGY201812006.htm -







 下载:
下载: