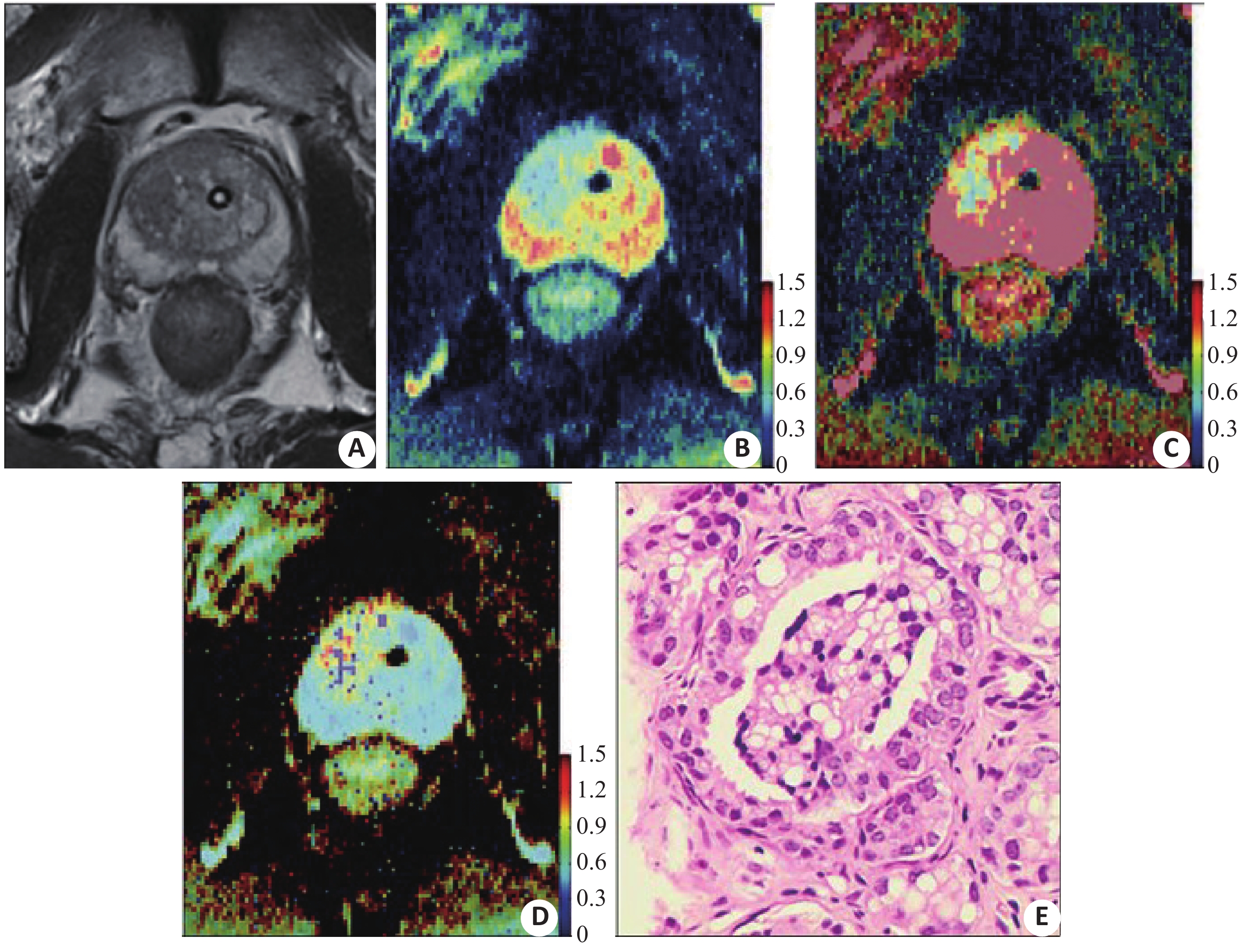
Diffusion kurtosis imaging combined with DWI at 3.0 T MRI for detection and assessment of aggressiveness of prostate cancer
-
摘要:
目的探讨3.0 T磁共振扩散峰度成像联合扩散加权成像在侵袭性前列腺癌诊断中的价值。 方法回顾性分析2015年5月~2019年6月我院经前列腺穿刺活检确诊并行磁共振扩散峰度成像和扩散加权成像进行诊断的患者共80例,包括49例侵袭性前列腺癌患者和31例良性前列腺增生患者。依据Gleason评分(GS)将侵袭性前列腺癌患者分为低级别组(GS≤3+3)和中、高级别组(GS≥3+4);获得DKI表观峰度系数(Kapp)和扩散加权成像表观扩散系数(ADC)。比较侵袭性前列腺癌与良性前列腺增生之间Kapp和ADC的差异,以及扩散峰度成像和扩散加权成像参数与Gleason评分的相关性,绘制ROC曲线评估二者诊断效能。 结果49例侵袭性前列腺癌患者共绘制肿瘤感兴趣区65个,包括低级别26个,中、高级别39个。相比低级别侵袭性前列腺癌和良性前列腺增生,中、高级别侵袭性前列腺癌的ADC值较低,Kapp值较高(P<0.01)。诊断侵袭性前列腺癌和对其进行分级时,Kapp的曲线下面积均小于ADC(0.947 vs 0.978,P<0.001;0.689 vs 0.894,P=0.008);二者联合诊断侵袭性前列腺癌和对其进行分级时,曲线下面积大于Kapp(0.979 vs 0.947,P=0.013;0.934 vs 0.689,P<0.001),二者联合诊断和分级的曲线下面积大于ADC,但差异无统计学意义(P>0.05);二者联合对侵袭性前列腺癌分级的特异性较单独采用Kapp要高(0.838 vs 0.730,P=0.035)。 结论ADC和Kapp参数均可用于诊断和评估侵袭性前列腺癌的侵袭性,扩散峰度成像联合扩散加权成像诊断和评估侵袭性前列腺癌的侵袭性并没有表现出比单独应用扩散加权成像具有更高的诊断效能。 Abstract:ObjectiveTo investigate the value of diffusion kurtosis imaging (DKI) combined with DWI in diagnosis and assessment of aggressiveness of prostate cancer (PCa). MethodsWe retrospectively analyzed 80 patients from May 2015 to June 2019 underwent DKI and MR images and confirmed by prostate biopsy, including 49 patients with PCa and 31 patients with benign prostatic hyperplasia (BPH). The patients with PCa were divided into a low-grade PCa group (Gleason score≤3+3) and intermediate- and high-grade PCa group (GS≥3+4). DKI-derived parameter (apparent kurtosis coefficient, Kapp) and a DWI-derived parameter (apparent diffusion coefficient, ADC) were obtained. The differences of Kapp and ADC between PCa and BPH, the correlation between DKI and DWI parameters and Gleason score were compared. ROC curves were drawn to evaluate the diagnostic efficacy. Results65 ROIs in 49 patients with PCa were drawn, including ROIs in 26 low-grade tumors and ROIs in 39 intermediate- and high-grade tumors. PCa and intermediate- and high-grade PCa had significantly lower ADC values and higher Kapp values than BPH and low-grade PCa (all P<0.01). The AUCs of Kapp were significantly lower than the AUCs of ADC in the diagnosis (0.947 vs 0.978, P<0.001) and grading (0.689 vs 0.894, P=0.008) of PCa. The AUCs of the combination of the two metrics were significantly higher than the AUCs of Kapp for the diagnosis (0.979 vs 0.947, P=0.013) and grading (0.934 vs 0.689, P<0.001) of PCa, and they were higher than the AUCs of ADC without significance between groups (P>0.05). The combination of the two metrics significantly increased the specificity in grading of PCa compared with Kapp alone (0.838 vs 0.730, P=0.035). ConclusionBoth ADC and Kapp can be used as quantitative parameters in detection and assessment of aggressiveness of PCa. The combination of DKI and DWI has no significant superiority to DWI alone in detection and assessment of the aggressiveness of PCa. -
Key words:
- diffusion kurtosis imaging /
- DWI /
- MRI /
- prostate cancer
-
表 1 ADC和Kapp值比较
Table 1. Comparison of ADC and Kapp
组别 ADC(×10−3 mm2/s) Kapp 前列腺增生 0.846±0.101 0.788±0.108 前列腺癌 0.557±0.095 1.079±0.152 P <0.001 <0.001 GS≤3+3 0.634±0.084 1.030±0.130 GS≥3+4 0.504±0.058 1.113±0.157 P <0.001 0.009 外周带前列腺癌 0.539±0.080 1.084±0.142 移行带前列腺癌 0.570±0.102 1.075±0.167 P 0.129 0.803 ADC: 表观弥散系数; Kapp: 表观峰度系数; GS: Gleason评分. 表 2 ADC、Kapp及联合诊断效能比较
Table 2. Comparison of ADC, Kapp and combined diagnostic efficacy
参数 AUC(95%CI) 截断值(×10−3 mm2/s) 敏感性 特异性 前列腺癌 vs 前列腺增生 ADC 0.978(0.960−0.997) 0.695* 0.925 0.922 Kapp 0.947(0.914−0.980) 0.905 0.911 0.868 ADC+Kapp 0.979(0.960−0.997) 0.922 0.943 GS≤3+3 vs GS≥3+4 ADC 0.894(0.813−0.974) 0.555* 0.892 0.868 Kapp 0.689(0.577−0.802) 1.055 0.679 0.730 ADC+Kapp 0.934(0.885−0.982) 0.906 0.838 -
[1] Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: a review[J]. Scand J Public Health, 2018, 46(1): 27-36. doi: 10.1177/1403494817715400 [2] Tyson MD, Penson DF, Resnick MJ. The comparative oncologic effectiveness of available management strategies for clinically localized prostate cancer[J]. Urol Oncol, 2017, 35(2): 51-8. doi: 10.1016/j.urolonc.2016.03.021 [3] Catala V, Vilanova JC, Gaya JM, et al. Multiparametric magnetic resonance imaging and prostate cancer: what's new[J]. Radiologia, 2017, 59(3): 196-208. doi: 10.1016/j.rx.2016.12.003 [4] Barbieri S, Bronnimann M, Boxler S, et al. Differentiation of prostate cancer lesions with high and with low Gleason score by diffusion-weighted MRI[J]. Eur Radiol, 2017, 27(4): 1547-55. doi: 10.1007/s00330-016-4449-5 [5] Sprinkart AM, Marx C, Traber F, et al. Evaluation of Exponential ADC (eADC) and computed DWI (cDWI) for the detection of prostate cancer[J]. Rofo, 2018, 190(8): 758-66. doi: 10.1055/a-0637-9980 [6] Luo M, Zhang L, Jiang XH, et al. Intravoxel incoherent motion: application in differentiation of hepatocellular carcinoma and focal nodular hyperplasia[J]. Diagn Interv Radiol, 2017, 23(4): 263-71. doi: 10.5152/dir.2017.16595 [7] Roethke MC, Kuder TA, Kuru TH, et al. Evaluation of diffusion kurtosis imaging versus standard diffusion imaging for detection and grading of peripheral zone prostate cancer[J]. Invest Radiol, 2015, 50(8): 483-9. doi: 10.1097/RLI.0000000000000155 [8] Hectors SJ, Semaan S, Song C, et al. Advanced diffusion-weighted imaging modeling for prostate cancer characterization: correlation with quantitative histopathologic tumor tissue composition-a hypothesis-generating study[J]. Radiology, 2018, 286(3): 918-28. doi: 10.1148/radiol.2017170904 [9] Hoang DA, Melodelima C, Souchon R, et al. Quantitative analysis of prostate multiparametric MR images for detection of aggressive prostate cancer in the peripheral zone: a multiple imager study[J]. Radiology, 2016, 280(1): 117-27. doi: 10.1148/radiol.2016151406 [10] Jambor I, Pesola M, Merisaari H, et al. Relaxation along fictitious field, diffusion-weighted imaging, and T2 mapping of prostate cancer: Prediction of cancer aggressiveness[J]. Magn Reson Med, 2016, 75(5): 2130-40. doi: 10.1002/mrm.25808 [11] Wang X, Tu N, Qin T, et al. Diffusion kurtosis imaging combined with DWI at 3-T MRI for detection and assessment of aggressiveness of prostate cancer[J]. Am J Roentgenol, 2018, 211(4): 797-804. doi: 10.2214/AJR.17.19249 [12] Suo S, Chen X, Wu L, et al. Non-Gaussian water diffusion kurtosis imaging of prostate cancer[J]. Magn Reson Imaging, 2014, 32(5): 421-7. doi: 10.1016/j.mri.2014.01.015 [13] Rosenkrantz AB, Sigmund EE, Winnick A, et al. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants[J]. Magn Reson Imaging, 2012, 30(10): 1534-40. doi: 10.1016/j.mri.2012.04.020 [14] Suo S, Chen X, Ji X, et al. Investigation of the non-Gaussian water diffusion properties in bladder cancer using diffusion kurtosis imaging: a preliminary study[J]. J Comput Assist Tomogr, 2015, 39(2): 281-5. doi: 10.1097/RCT.0000000000000197 [15] Filli L, Wurnig M, Nanz D, et al. Whole-body diffusion kurtosis imaging: initial experience on non-Gaussian diffusion in various organs[J]. Invest Radiol, 2014, 49(12): 773-8. doi: 10.1097/RLI.0000000000000082 [16] Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging[J]. J Urol, 2017, 197(2s): 134-9. [17] Kozal S, Peyronnet B, Cattarino S, et al. Influence of pathological factors on oncological outcomes after robot-assisted radical prostatectomy for localized prostate cancer: results of a prospective study[J]. Urol Oncol, 2015, 33(7): 330-7. [18] Gillessen S, Attard G, Beer T M, et al. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference 2017[J]. Eur Urol, 2018, 73(2): 178-211. doi: 10.1016/j.eururo.2017.06.002 [19] Salami SS, Ben-Levi E, Yaskiv O, et al. Risk stratification of prostate cancer utilizing apparent diffusion coefficient value and lesion volume on multiparametric MRI[J]. J Magn Reson Imaging, 2017, 45(2): 610-6. doi: 10.1002/jmri.25363 [20] Chen Y, Ren W, Zheng D, et al. Diffusion kurtosis imaging predicts neoadjuvant chemotherapy responses within 4 days in advanced nasopharyngeal carcinoma patients[J]. J Magn Reson Imaging, 2015, 42(5): 1354-61. doi: 10.1002/jmri.24910 [21] Tamada T, Sone T, Jo Y, et al. Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade[J]. J Magn Reson Imaging, 2008, 28(3): 720-6. doi: 10.1002/jmri.21503 [22] Shaikhibrahim Z, Lindstrot A, Ellinger J, et al. The peripheral zone of the prostate is more prone to tumor development than the transitional zone: is the ETS family the key[J]. Mol Med Rep, 2012, 5(2): 313-6. [23] Tamada T, Prabhu V, Li J, et al. Prostate cancer: diffusion-weighted MR imaging for detection and assessment of aggressiveness-comparison between conventional and kurtosis models[J]. Radiology, 2017, 284(1): 100-8. doi: 10.1148/radiol.2017162321 -







 下载:
下载: