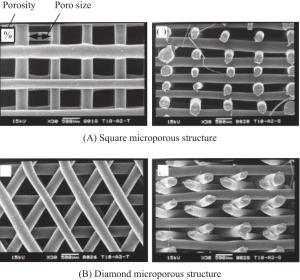
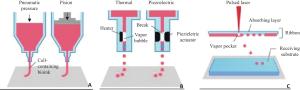
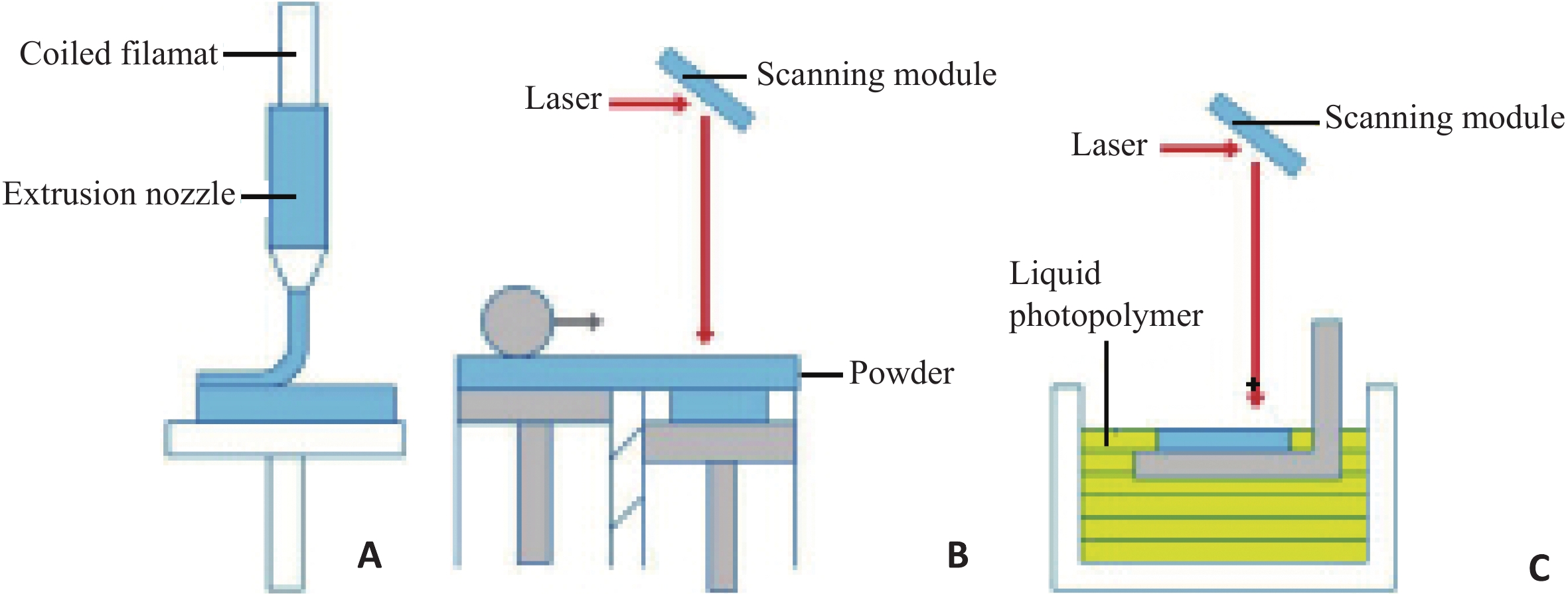
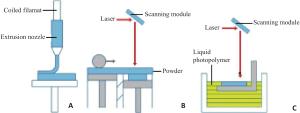
Research progress in microporous structure and poroisity of 3D bio-printing scaffolds
-
摘要: 近年来,3D生物打印受到越来越多研究者的关注,该技术弥补了传统组织工程技术的局限性,它可打印出精准、个性化的支架结构,并可以任意地设计形状、尺寸、孔的结构和孔隙率等。3D生物打印支架在组织工程应用中通常提供结构支撑和适宜的微环境以支持细胞行为,而支架的微孔结构及孔隙率对细胞的行为和健康至关重要,所以3D生物打印支架应该是具有高度多孔和互连的孔网络结构,以促进营养运输、废物排泄和细胞生长增殖分化。本文将综述从无生命到有生命的3D生物打印支架材料和打印方式,以及着重介绍支架的微孔结构及孔隙率相关研究。Abstract: In recent years, 3D bio-printing has attracted more and more attention from researchers. This technology makes up for the limitations of traditional tissue engineering technology. It can print out accurate and personalized scaffold structure, and can design shape, size, pore structure and porosity at etc. The 3D bio-printing scaffold usually provides structural support and suitable microenvironment to support cell behavior in tissue engineering. The microporous structure and porosity of the scaffold are essential to the behavior and health of the cells. Therefore, the 3D bio-printing scaffolds should be highly porous and interconnected pore networks to facilitate nutrient transportation, waste excretion, and cell growth、 proliferation and differentiation. This paper reviewed the materials and printing methods of 3D bio-printing scaffolds from inanimate to life, and focued on the study of the microporous structure and porosity of the scaffolds.
-
Key words:
- 3D bio-printing /
- scaffolds /
- microporous structure /
- porosity
-
表 1 不同细胞类型活性所需的各种支架的孔径和孔隙率
细胞类型 支架材料 孔径(μm) 孔隙率(%) 参考 小鼠胚胎成纤维细胞 胶原/海藻酸钠纤维凝胶 150~300 90 [24] 小鼠成骨细胞 氧化石墨烯(GO)-藻酸盐-壳聚糖-胶原(GO-SA-CS-Col) 75~250 78 [25] 大鼠间充质干细胞 胶原蛋白 200~700 65~90 [26] 人间充质干细胞 聚氨酸/透明质酸 300~700 - [27] 大鼠骨髓间充质干细胞 13-93生物活性玻璃/海藻酸钠 500 65~87 [28] 人骨肉瘤细胞 壳聚糖硫醇聚合物 100~350 90 [29] 人皮肤成纤维细胞 壳聚糖 52~88 80 [30] 小鼠胚胎细胞 二醛纤维素纳米晶体/明胶 450~500 95.14 [31] 兔软骨细胞 壳聚糖/聚己内酯/II型胶原 100~300 75 [32] 雌性小鼠卵泡 明胶 250~350 - [8] 大鼠骨髓间充质干细胞 聚-β-羟丁酸 500~700 55.8 [33] 骨髓间充质干细胞 明胶/聚乳酸/聚己内酯 100 80 [34] 人间充质干细胞 聚己内酯 245~433 49~57 [19] 鼠胚胎成纤维细胞 聚己内酯 20~70 80 [35] 大鼠间充质干细胞 聚己内酯 100~350 90 [36] 纤人包皮成维细胞 丝素蛋白 200~250 86 [37] 人软骨细胞 丝素蛋白 100~300 - [38] 人间充质干细胞 丝素蛋白 204.7~226.5 75.8~84.4 [39] 表 2 两类生物材料3D打印支架的比较
分类 无生命的3D生物打印支架 有生命的3D生物打印支架 打印方式 熔融沉积成形;选择性激光烧结;光固化立体印刷 挤压式生物打印;喷墨细胞生物打印;激光辅助生物打印 支架材料 金属(钛合金、钴铬合金、不锈钢和铝合金等);陶瓷(氧化铝、生物活性玻璃和磷酸钙);高分子聚合物 活细胞混合凝胶类材料(胶原蛋白,明胶,藻酸盐,壳聚糖和透明质酸等) 优点 机械性能好;高效;低消耗;低成本 仿生;细胞浓度可控且分布均匀;可降解;应用于软组织 缺点 难以精确控制支架的孔形态、孔径和总孔隙率;需要后期加工处理;接种在支架上的细胞分布不均;耗时 黏度低,机械强度差;细胞技术要求高;生物材料选择种类少;制造平台要求无菌 -
[1] Yoo D. New paradigms in hierarchical porous scaffold design for tissue engineering[J]. Mater Sci Engin Mater Biolog Applicat, 2013, 33(3): 1759-72 doi: 10.1016/j.msec.2012.12.092 [2] Li J, Chen M, Fan X, et al. Recent advances in bioprinting techniques: approaches, applications and future prospects[J]. J Translat Med, 2016, 14(1): 271-5 doi: 10.1186/s12967-016-1028-0 [3] Do A, Khorsand B, Geary SM, et al. 3D Printing of Scaffolds for Tissue Regeneration Applications[J]. Advanced Healthcare Materials, 2015, 4(12): 1742-62 doi: 10.1002/adhm.v4.12 [4] 石然. 基于细胞3D打印技术的肿瘤模型构建研究[D]. 杭州: 杭州电子科技大学, 2015. [5] Zein I, Hutmacher DW, Tan KC, et al. Fused deposition modeling of novel scaffold architectures for tissue engineering applications[J]. Biomaterials, 2002, 23(4): 1169-85 doi: 10.1016/S0142-9612(01)00232-0 [6] Aliabouzar M, Lee SJ, Zhou X, et al. Effects of scaffold microstructure and low intensity pulsed ultrasound on chondrogenic differentiation of human mesenchymal stem cells[J]. Biotechnol Bioengin, 2018, 115(2): 495-506 doi: 10.1002/bit.26480 [7] Danilevicius P, Georgiadi L, Pateman CJ, et al. The effect of porosity on cell ingrowth into accurately defined, laser-made, polylactide-based 3D scaffolds[J]. Applied Surf Sci, 2015, 336(1): 2-10 [8] Laronda MM, Rutz AL, Xiao S, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice[J]. Nature Commun, 2017, 8(13): 15261-7 [9] Munoz-Abraham AS, Rodriguez-Davalos MI, Bertacco A, et al. 3D Printing of organs for transplantation: where are we and where are we heading[J]. Current Transplant Rep, 2016, 3(1): 93-9 doi: 10.1007/s40472-016-0089-6 [10] 吴明明, 林子锋, 程德林, 等. 生物打印技术在组织与器官修复中的应用进展[J]. 集成技术, 2018, 25(1): 33-8 [11] Datta P, Ayan B, Ozbolat IT. Bioprinting for vascular and vascularized tissue biofabrication[J]. Acta Biomaterialia, 2017, 51(1): 1-20 [12] Desrus H, Chassagne B, Moizan F, et al. Effective parameters for film-free femtosecond laser assisted bioprinting[J]. Applied Optics, 2016, 55(14): 3879-85 doi: 10.1364/AO.55.003879 [13] Luo F. The study on biomedical materials of hydrogel[J]. Guangdong Chem Ind, 2011, 18(2): 217-22 [14] 罗文峰, 杨雪香, 敖宁建. 生物医用材料的3D打印技术与发展[J]. 材料导报, 2016, 30(13): 81-6 [15] Ji S, Guvendiren M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs[J]. Front Bioengin Biotechnol, 2017, 5(23): 3106-14 [16] 石然, 徐铭恩, 周青青, 等. 基于细胞3D打印技术的体外肿瘤模型构建研究[J]. 中国生物医学工程学报, 2015, 34(5): 618-22 doi: 10.3969/j.issn.0258-8021.2015.05.014 [17] Lee VK, Kim DY, Ngo H, et al. Creating perfused functional vascular channels using 3D bio-printing technology[J]. Biomaterials, 2014, 35(28): 8092-102 doi: 10.1016/j.biomaterials.2014.05.083 [18] Fan C, Wang DA. Macroporous hydrogel scaffolds for three-dimensional cell culture and tissue engineering[J]. Tissue Engineer Part B Reviews, 2017, 23(5): 451-6 doi: 10.1089/ten.teb.2016.0465 [19] Domingos M, Intranuovo F, Russo T, et al. The first systematic analysis of 3D rapid prototyped poly(Îμ-caprolactone) scaffolds manufactured through BioCell printing: the effect of pore size and geometry on compressive mechanical behaviour and in vitro hMSC viability[J]. Biofabrication, 2013, 5(4): 045004-12 doi: 10.1088/1758-5082/5/4/045004 [20] Billiet T, Gevaert E, De ST, et al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability[J]. Biomaterials, 2014, 35(1): 49-62 doi: 10.1016/j.biomaterials.2013.09.078 [21] Luo T, Tang L, Yang YD, et al. Construction of multilayered hydrogel structure of rabbit primary hepatocytes using 3D bioprinting technique[J]. Chin J Health Laborat Technol, 2017, 52(8): 903-10 [22] Fan C, Wang DA. Effects of permeability and living space on cell fate and neo-tissue development in hydrogel-based scaffolds: a study with cartilaginous model[J]. Macromol Biosc, 2015, 15(4): 535-45 doi: 10.1002/mabi.v15.4 [23] You F, Wu X, Zhu N, et al. 3D Printing of porous cell-laden hydrogel constructs for potential applications in cartilage tissue engineering[J]. Acs Biomat Sci ngin, 2016, 2(7): 1200-10 doi: 10.1021/acsbiomaterials.6b00258 [24] Lin S, Dongmei L, Songmei X, 等. 以胶原-海藻酸钠纤维凝胶为原料的具有潜在组织工程应用的仿生支架材料的制备与评价[J]. 西部皮革, 2014, 13(6): 34-42 doi: 10.3969/j.issn.1671-1602.2014.06.011 [25] Kolanthai E, As P, Khajuria DK, et al. Graphene Oxide-A tool for preparation of chemically crosslinking free alginate-chitosan-collagen scaffold for bone tissue engineering[J]. Acs Appl Mater Interfaces, 2018, 10(15): 12441-52 doi: 10.1021/acsami.8b00699 [26] Wu YJ, Chen T, Chen IF, et al. Developing highly porous collagen scaffolds by using alginate microsphere porogens for stem cell cultures[J]. Materials Letters, 2018, 223(2): 120-3 [27] Hung KC, Tseng CS, Dai LG, et al. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering[J]. Biomaterials, 2016, 83(2): 156-68 [28] Luo G, Ma Y, Cui X, et al. 13-93 bioactive glass/alginate composite scaffolds 3D printed under mild conditions for bone regeneration[J]. Rsc Advances, 2017, 32(10): 11880-9 [29] Borsagli FG, Carvalho IC, Mansur HS. Amino acid-grafted and N- acylated chitosan thiomers: Construction of 3D bio-scaffolds for potential cartilage repair applications[J]. Inter J Biolog Macromolec, 2018, 114(2): 270-82 [30] Ji C, Shi J. Thermal-crosslinked porous chitosan scaffolds for soft tissue engineering applications[J]. Materials Sci Engin Material Biolog Applic, 2013, 33(7): 3780-5 doi: 10.1016/j.msec.2013.05.010 [31] Jiang Y, Zhou J, Yang Z, et al. Dialdehyde cellulose nanocrystal/gelatin hydrogel optimized for 3D printing applications[J]. J Materials Sci, 2018, 53(16): 11883-900 doi: 10.1007/s10853-018-2407-0 [32] Zhu Y, Wan Y, Zhang J, et al. Manufacture of layered collagen/chitosan-polycaprolactone scaffolds with biomimetic microarchitecture[J]. Colloids Surf B Biointerfaces, 2014, 113(3): 352-60 [33] Saska S, Pires LC, Cominotte MA, et al. Three-dimensional printing and in vitro evaluation of poly(3-hydroxybutyrate) scaffolds functionalized with osteogenic growth peptide for tissue engineering[J]. Materials Sci Engin C, 2018, 89(2): 265-73 [34] Shahrezaee M, Salehi M, Keshtkari S, et al. In vitro and in vivo investigation of PLA/PCL scaffold coated with metformin-loaded gelatin nanocarriers in regeneration of critical-sized bone defects[J]. Nanomedic Nanotechnol Biol Med, 2018, 14(7): 2061-73 doi: 10.1016/j.nano.2018.06.007 [35] Visscher LE, Dang HP, Knackstedt M, et al. Novel 3D printed Polycaprolactone scaffolds with dual macro-microporosity for applications in local delivery of antibiotics[J]. Materials Sci Engin C Materials Biolog Applicat, 2018, 87(1): 78-84 [36] Yoshimoto H, Shin YM, Terai H, et al. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering[J]. Biomaterials, 2003, 24(12): 2077-82 doi: 10.1016/S0142-9612(02)00635-X [37] Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds[J]. Biomaterials, 2009, 30(15): 2956-65 doi: 10.1016/j.biomaterials.2009.02.006 [38] Zhou F, Zhang X, Cai D, et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair[J]. Acta Biomaterialia, 2017, 63(1): 64-75 [39] Warnecke D, Stein S, et al. Biomechanical, structural and biological characterisation of a new silk fibroin scaffold for meniscal repair[J]. Mech Behav Biomed Mater, 2018, 86(3): 314-24 -






 下载:
下载: