Value of MR dynamic enhanced combined diffusion-weighted imaging in differential diagnosis of benign and malignant tumors of parotid gland
-
摘要:
目的探讨MR动态增强扫描(DCE-MRI)与扩散加权成像(DWI)联合应用对鉴别腮腺良恶性肿瘤的诊断价值。 方法回顾性分析40例经手术病理证实的腮腺肿瘤患者,年龄28~80岁(平均46岁),男性25例,女性15例,术前均行MR平扫、动态增强和DWI扫描,绘制肿瘤的动态增强时间-信号强度曲线(TIC)并获得相关参数(峰值时间Tpeak、600 s时的廓清率),在表观弥散参数(ADC)图上显示测量的肿瘤ADC值,比较良恶性腮腺肿瘤之间的TIC参数及ADC值,并分析多形性腺瘤、腺淋巴瘤与恶性肿瘤之间上述参数的差异。 结果40例腮腺肿瘤中,良性28例,主要为多形性腺瘤和腺淋巴瘤;恶性12例,病理类型较多。多形性腺瘤以A型(缓慢上升型)、C型(快进缓降型)曲线为主,且A型曲线只存在于多形性腺瘤中,其中1例为多形性腺瘤恶变,可将多形性腺瘤与其他良性肿瘤及恶性肿瘤进行鉴别;腺淋巴瘤以B型(速升速降型)曲线为主,较具有特征性,恶性肿瘤以C型曲线为主,但是C型曲线也存在于部分良性肿瘤中,两者之间存在部分重叠。腮腺良性肿瘤的ADC值高于恶性肿瘤的ADC值(1.13×10-3 mm2/s vs 0.84×10-3 mm2/s),其中多形性腺瘤和恶性肿瘤、腺淋巴瘤的ADC值差异有统计学意义(P < 0.01),但腺淋巴瘤和恶性肿瘤的ADC值差异无统计学意义(P>0.05)。 结论MR常规扫描结合动态增强为腮腺肿瘤的定性诊断提供有价值信息,联合ADC值可进一步提高对良恶性肿瘤的鉴别诊断效能。 Abstract:ObjectiveTo explore the diagnostic value of the combined application of magnetic resonance dynamic enhanced scanning (DCE-MRI) and diffusion weighted imaging (DWI) in the differentiation of benign and malignant tumors of the parotid gland. MethodsForty patients with parotid gland tumors confirmed by surgical pathology were retrospectivly analyzed. The patients aged from 28 to 80 years old (average age at 46 years old), with 25 males and 15 females. The patients were performed MR plain scan, dynamic enhancement and DWI scan before surgery to draw tumors. The dynamic enhancement time-signal intensity curve (TIC) and related parameters (peak time Tpeak, clearance rate WR at 600 s) were displayed, and the measured ADC value of the tumor was displayed on the ADC map. Two independent sample t tests were used to compare benign and malignant parotid tumors. Differences between TIC parameters and ADC values were analyzed by single-factor analysis of variance for pleomorphic adenoma, adenolymphoma, and malignant tumors. ResultsTwenty-eight of the 40 cases of parotid gland tumors were benign, mainly pleomorphic adenoma and adenolymphoma, and 12 were malignant with many pathological types. Polymorphic adenoma was dominated by A-type (slow-rising type) and C-type (fastforward slow- declining type) curves, and the A-type curve only existed in pleomorphic adenoma, of which 1 case was malignant pleomorphic adenoma. Distinguish pleomorphic adenoma from other benign tumors and malignant tumors; adenolymphomas were dominated by B-type (rapid ascent and descent) curves, which were more characteristic. Malignant tumors were dominated by C-type curves, but it also existed in some benign tumors. There was a partial overlap between them. The ADC value of benign tumors of the parotid gland was significantly higher than that of malignant tumors (1.13× 10-3 mm2/s vs 0.84 × 10-3 mm2/s). The differences of ADC values between pleomorphic adenoma, malignant tumor, and adenolymphoma were significant (P < 0.01). The difference of ADC values between adenolymphoma and malignant tumor was not significant (P>0.05). ConclusionMR routine scanning combined with dynamic enhancement provides valuable information for the qualitative diagnosis of parotid gland tumors. Combined with ADC value can further improve the differential diagnosis of benign and malignant tumors. -
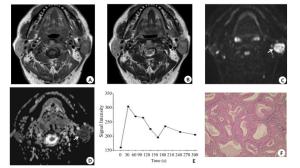
图 1 多形性腺瘤的MRI表现与病理结果
女, 37岁, 右侧腮腺浅叶多形性腺瘤(箭头所示), 境界清晰.横断面T1WI(A)呈均匀稍低信号, 横断面T2WI(B)呈高信号; 横断面T1WI增强扫描(C)示病灶欠均匀强化,病灶内感兴趣区(椭圆所示)测得ADC值为1.67×10-3mm2/s(D), TIC(E)表现为A型缓慢上升型曲线, Tpeak=180 s; 肿块术后病理(F)显示:镜下可见黏液液化区及导管上皮样成分, 分化良好, 未见软骨及鳞状上皮(HE, 放大倍数: ×100)
Figure 1. MRI performance and pathological results of pleomorphic adenoma
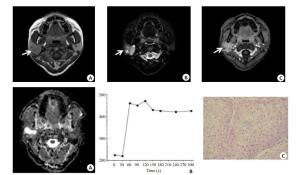
图 2 腮腺腺淋巴瘤的MRI表现及病理结果
男, 62岁, 左侧腮腺腺淋巴瘤(箭头); MRI示肿块边缘清楚, 包膜完整, T1WI及T2WI均为中等信号(A, B), 内部少许斑片状稍高信号; DWI显示病灶呈不均匀高信号(C), 图像后处理测得ADC=0.8×10-3 mm2/s(D), TIC表现为B型快进快退型曲线(E), Tpeak=30 s, WR为71%;病理(F)显示:镜下可见肿物由被覆上皮样细胞和淋巴组织组成, 上皮样细胞浆嗜酸性, 部分围绕淋巴组织形成乳头状结构, 部分组织呈囊样结构(HE, ×200)
Figure 2. MRI manifestations and pathological results of parotid gland lymphoma
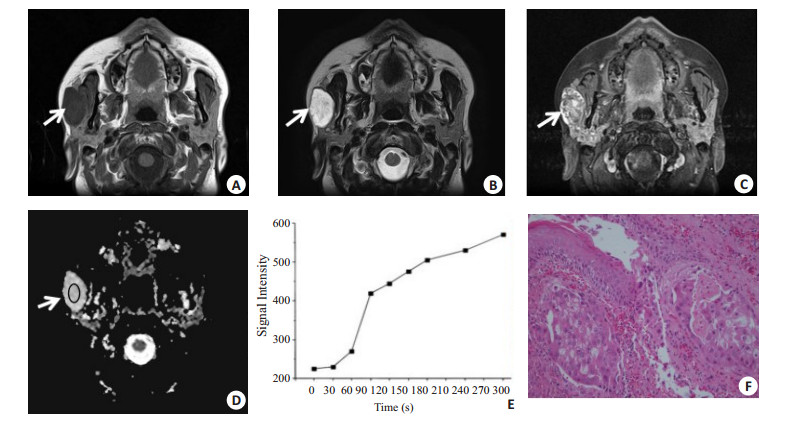
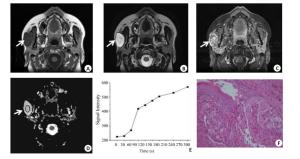
图 3 腮腺恶性肿瘤的MRI表现与病理结果
男, 70岁, 右侧腮腺黏液表皮样癌, 呈浅分叶(箭头).MR示T1WI呈等信号, T2WI脂肪抑制序列为高信号, 边界尚清, 内部可见小囊变(A, B); 增强扫描实质部分较明显强化(C), 测得ADC值为1.25×10-3mm2/s(D), TIC表现为C型快进缓降型曲线(E), Tpeak=60 s, WR为21%;病理(F)显示具有高核质比的纺锤形细胞, 还可以看到大量的纤维基质组织和腺体形成(HE, ×40)
Figure 3. MRI findings and pathological results of parotid gland malignancies
表 1 腮腺良恶性肿瘤的一般资料比较
Table 1. Comparison of general data of benign and malignant tumors of parotid gland
一般资料 良性肿瘤组(n=28) 恶性肿瘤组(n=12) P 男/女(n) 18/10 7/5 0.780 年龄(岁, Mean±SD) 48.63±14.5 53.74±16.8 0.354 单/双侧或多发(n) 24/4 11/1 0.632 最大径(cm, Mean±SD) 2.53±1.376 3.349±1.151 0.826 表 2 腮腺良恶性肿瘤的TIC曲线分布情况(n)
Table 2. TIC curve distribution of benign and malignant tumors of the parotid gland
病理类型 n 曲线类型 A B C D 多形性腺瘤 16 9 0 1 6 腺淋巴瘤 10 0 8 2 0 基底细胞瘤 1 0 0 0 1 肌上皮瘤 1 0 0 1 0 黏液表皮样癌 3 0 1 0 2 腺样囊腺癌 2 0 0 0 2 腺泡细胞癌 1 0 0 0 1 基底细胞癌 1 0 0 0 1 淋巴上皮样癌 1 0 0 1 0 淋巴瘤 1 0 0 0 1 多形性腺瘤恶变 1 0 0 0 1 肌上皮癌 1 0 1 0 0 转移瘤 1 0 0 1 0 表 3 腮腺良恶性肿瘤MRI的ADC、Tpeak、WR值比较(Mean±SD)
Table 3. Comparison of ADC, Tpeak and WR values of MRI between benign and malignant tumors of parotid gland
肿瘤类型 ADC (×10-3mm2/s) Tpeak(s) WR (%) 良性肿瘤组(n=28) 1.15±0.32* 83.46±24.53 40.36±15.62* 多形性腺瘤(n=16) 1.45±0.24*# 256.31±118.21*# 4.72±1.81*# 腺淋巴瘤(n=10) 0.83±0.35 78.60±30.52 52.61±11.31* 其他(n=2) 1.16±0.42 52.42±36.10 22.42±3.52 恶性肿瘤组(n=12) 0.84±0.15 75.81±28.93 26.71±5.20 *P < 0.05 vs恶性肿瘤; #P < 0.05 vs腺淋巴瘤; ADC: apparent diffusion coefficient; Tpeak: Peak time; WR:Washout rate. -
[1] 杜立新, 元建鹏, 关弘, 等.腮腺恶性肿瘤的MRI诊断价值及其病理基础[J].南方医科大学学报, 2010, 30(5): 1107-10. http://d.old.wanfangdata.com.cn/Periodical/dyjydxxb201005056 [2] 曹海营, 金宇, 赵景新, 等.改良穿刺活检技术用于降低VX2肌肉肿瘤穿刺并发症的研究[J].中国全科医学, 2016, 19(5): 560-4. doi: 10.3969/j.issn.1007-9572.2016.05.015 [3] 巩鑫, 陶晓峰.动态增强磁共振在腮腺肿瘤诊断中的应用价值[D].上海: 上海交通大学, 2015 [4] 杨功鑫, 王平仲, 朱文静, 等.腮腺肿瘤的磁共振弥散加权成像评价[J].中国医学计算机成像杂志, 2013, 19(6):489-93. http://d.old.wanfangdata.com.cn/Periodical/zgyxjsjcx201306004 [5] 郑少燕, 曾向廷, 吴先衡, 等. 3.0TMR动态增强扫描半定量分析对腮腺肿块鉴别诊断的价值[J].临床放射学杂志, 2015, 34(3):346-50. [6] Uhl M, Altehoefer C, Kontny U, et al. MRI-diffusion imaging of neuroblastomas: first results and correlation to histology[J]. Eur Radiol, 2002, 12(9):2335-8. doi: 10.1007/s00330-002-1310-9 [7] Wang PZ, Yang J, Yu Q, et al. Evaluation of solid lesions affecting masticator space with diffusion-weighted MR imaging[J]. Oral Surg Oral MedOral Pathol Oral Radiol Endod, 2010, 109(6):900-7. doi: 10.1016/j.tripleo.2010.01.005 [8] Yabuuchi H, Matsuo Y, Kamitani T, et al. Parotid gland tumors: can addition of diffusion- weighted MR imaging to dynamic contrastenhanced MR imaging improve diagnostic accuracy in characterization[J]?Radiology, 2008, 249(3):909-16. doi: 10.1148/radiol.2493072045 [9] 胡涛, 刘琼, 郑晓林, 等.腮腺恶性肿瘤临床与影像学分析[J].影像诊断与介入放射学, 2014, 23(4):336-9. doi: 10.3969/j.issn.1005-8001.2014.04.013 [10] 方三高. WHO(2017)头颈部肿瘤分类[J].诊断病理学杂志, 2017, 24 (8):638-41. doi: 10.3969/j.issn.1007-8096.2017.08.024 [11] 刘彤华.诊断病理学[M].北京:人民卫生出版社, 2012. [12] 沈世华, 李建卫, 吴松松, 等.超声与MRI鉴别腮腺常见良恶性肿瘤的对比性研究及应用[J].医学影像学杂志, 2018, 28(10):1619-24. http://d.old.wanfangdata.com.cn/Periodical/yxyxxzz201810010 [13] 于冬洋, 单奔, 柳勇, 等.3.0T磁共振DWI及动态增强扫描在腮腺常见肿瘤中的诊断价值[J].中华全科医师杂志, 2018, 17(4):303-6. doi: 10.3760/cma.j.issn.1671-7368.2018.04.014 [14] 童娟, 胡春洪, 王小林, 等.扩散加权联合动态对比增强磁共振成像鉴别诊断腮腺良恶性肿瘤[J].中国医学影像技术, 2017, 33(8):1197- 201. http://d.old.wanfangdata.com.cn/Periodical/zgyxyxjs201708019 [15] Gandolfi MM, Slattery W 3rd. Parotid gland tumors and the facial nerve[J].Otolaryngol Clin North Am, 2016, 49(2):425-34. doi: 10.1016/j.otc.2015.12.001 [16] 俞顺, 石清磊, 苏家威, 等. DCE-MRI定量参数在不同病理类型腮腺肿瘤鉴别诊断中的初步研究[J].磁共振成像, 2017, 8(9):654-61. http://d.old.wanfangdata.com.cn/Periodical/cgzcx201709003 [17] 蒯新平, 陶晓峰.CT和MRI功能成像在腮腺肿瘤诊断中的研究[J].实用放射学杂志, 2011, 27(4):628-30. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syfsxzz201104044 [18] 郑宁, 王光彬, 齐先龙, 等. 3.0T磁共振DWI联合动态增强扫描在唾液腺肿瘤中的诊断价值[J].医学影像学杂志, 2016, 26(7):1193-8. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yxyxxzz201607014 [19] Eida S, Sumi M, Sakihama N, et al. Apparent diffusion coefficient mapping of salivary gland tumors: prediction of the benignancy and malignancy[J].AmJNeuroradiol, 2007, 28(1):116-21. [20] Motoori K, Yamamoto S, Ueda T, et al. Inter- and intratumoral variability in magnetic resonance imaging of pleomorphic adenoma: an attempt to interpret the variable magnetic resonance findings[J]. J ComputAssistTomogr, 2004, 28(2):233-46. doi: 10.1097-00004728-200403000-00014/ [21] Habermann CR, Arndt C, Graessner J, et al. Diffusion-weighted Echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible[J]? Am J Neuroradiol, 2009, 30(3):591-6. doi: 10.3174/ajnr.A1412 [22] Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging[J]. Magn Reson Med, 2000, 43(6):828-36. doi: 10.1002/1522-2594(200006)43:6<828::AID-MRM8>3.0.CO;2-P [23] Yoshino N, Yamada I, Ohbayashi N, et al. Salivary glands and lesions: evaluation of apparent diffusion coefficients with split-Echo diffusion-weighted MR imaging: initial results[J]. Radiology, 2001, 221(3):837-42. doi: 10.1148/radiol.2213010131 [24] Yerli H, Aydin E, Haberal N, et al. Diagnosing common parotid tumours with magnetic resonance imaging including diffusionweighted imaging vs fine-needle aspiration cytology: a comparative study[J].Dentomaxillofac Radiol, 2010, 39(6):349-55. doi: 10.1259/dmfr/15047967 [25] 张赞霞, 程敬亮, 张勇. DWI联合动态增强MRI鉴别诊断腮腺肿瘤良恶性[J].中国医学影像技术, 2014, 30(7):1015-8. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgyxyxjs201407016 [26] 王萍, 张成周, 王宁, 等.常规MRI联合DWI在腮腺常见肿瘤中的诊断价值[J].放射学实践, 2012, 27(4):378-81. doi: 10.3969/j.issn.1000-0313.2012.04.005 -






 下载:
下载: