Application of two-photon imaging on cell transplantation and therapy
-
摘要: 双光子成像技术具有活体三维深层成像的能力,是重要的活体成像工具,在针对生物组织相关的活体、原位研究中应用广泛。通过移植细胞建立研究模型,可以在真实的细胞微环境中进行过程与机理研究。结合双光子成像技术,可以对移植细胞进行在体形态学鉴定与功能评价,避免体外培养模型带来的差异。双光子成像技术促进了疾病模型的研究,为疾病与治疗等病理生理过程的研究提供了重要的研究手段。细胞移植已经用于中枢神经系统、心肌、骨髓以及抗肿瘤药物等研究模型建立中,本文对双光子成像技术的技术原理、应用领域进行综述,并探讨了该技术的发展前景。双光子成像技术以其较大的成像深度、较高的成像质量等特点,满足了在体成像的需求,在以动物为研究对象的研究中发挥了重要的作用,使我们对活细胞生理、病理和药理领域的认识得到极大的发展。Abstract: Two-photon imaging technique realizes in vivo 3D deep-tissue imaging. It is an important tool of in vivo imaging and applied in research on in vivo and in situ biological tissue broadly. Research model established by cell transplantation helps study of process and mechanism in the real microenvironment. Using two-photon imaging, morphological observation and functional evaluation about donor cells is realized in living tissue, eliminating differences in vitro tissue culture models. Research of disease models are promoted by utilizing two-photon imaging. This technique is an important tool for research on disease and therapy pathphysiologic events. Cell transplants are used to establish research models of central nervous system, myocardium, bone marrow and anticancer drug. This article reviews the application of research on these regions with two-photon imaging technique, and discusses the development of this technique.
-
Key words:
- two-photon imaging /
- cell transplantation /
- in vivo imaging
-
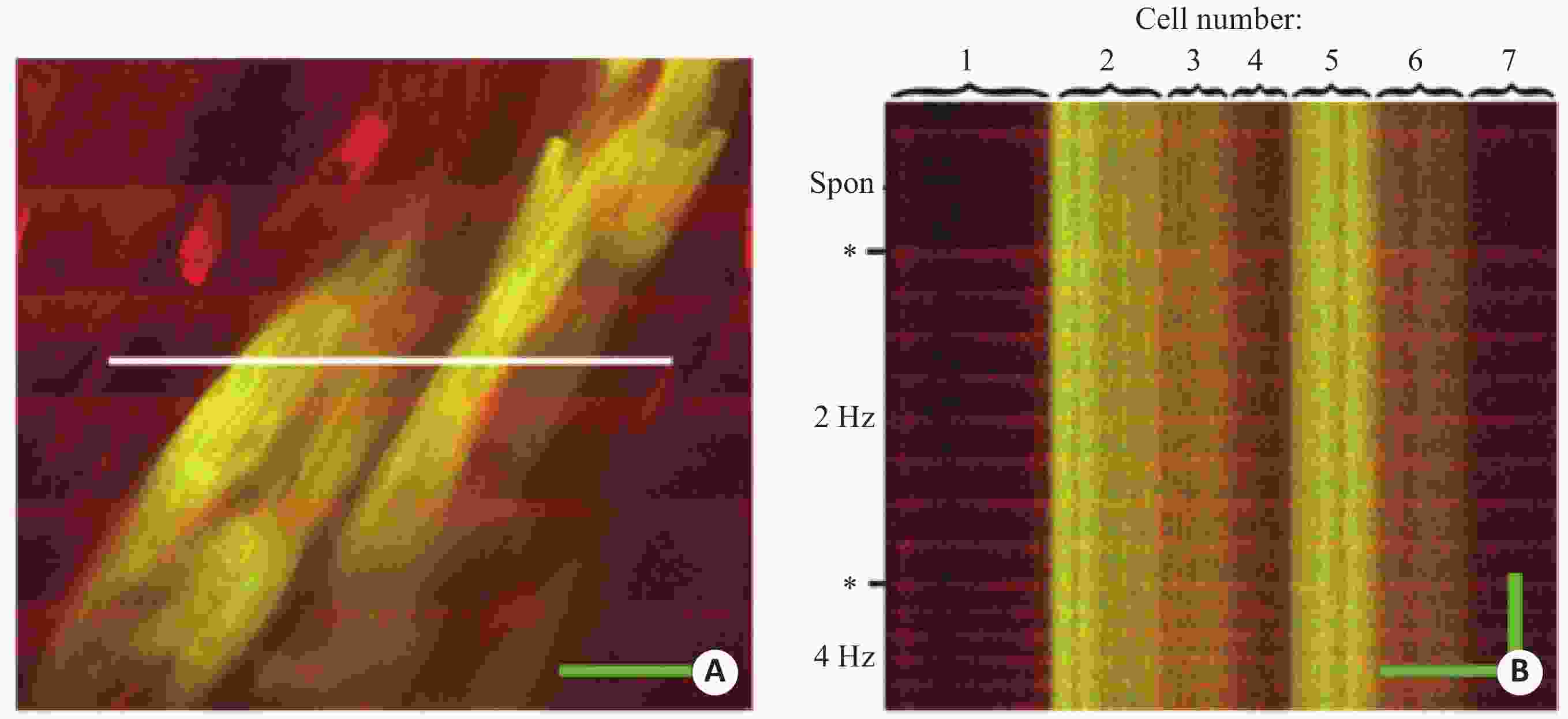
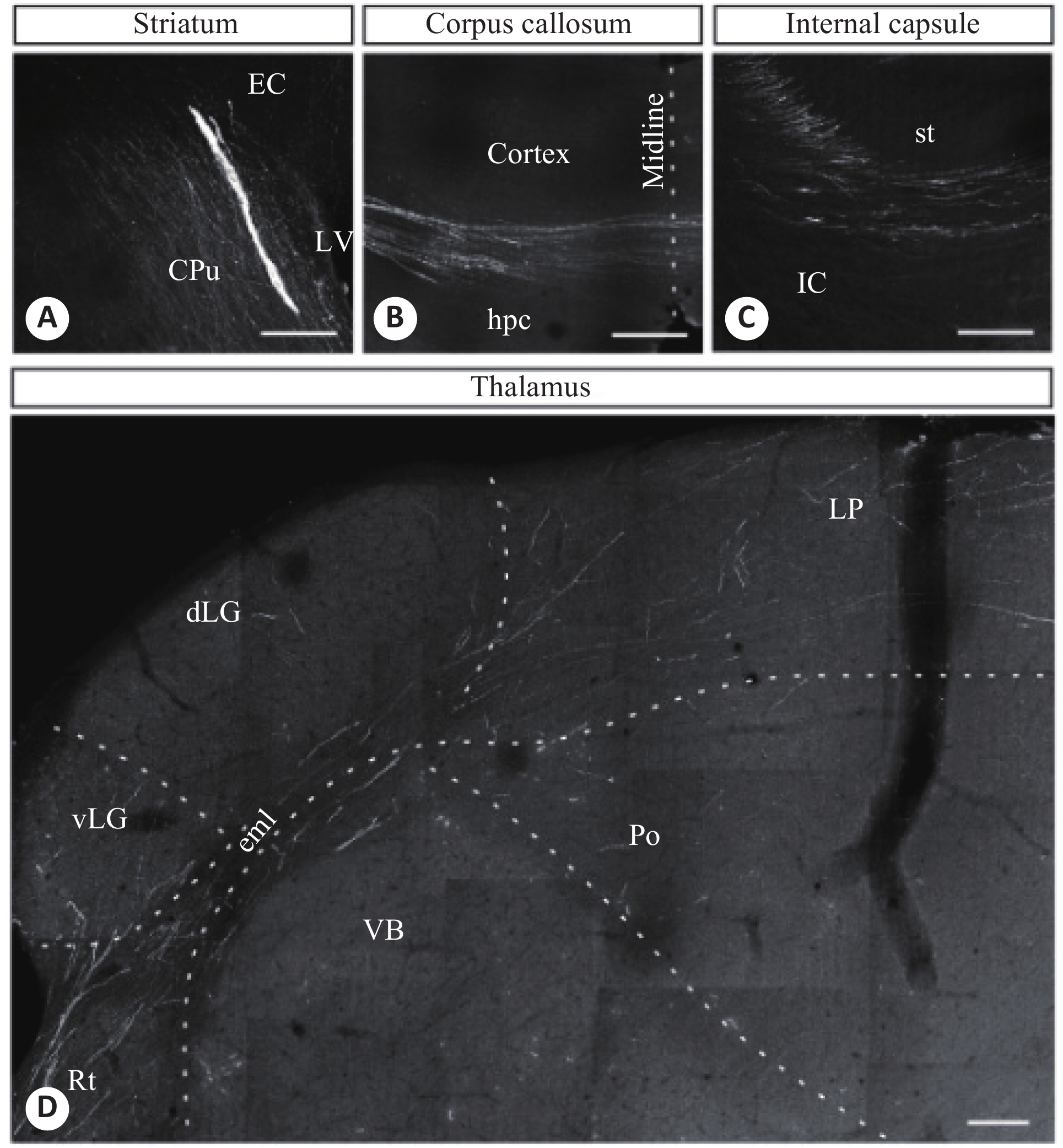
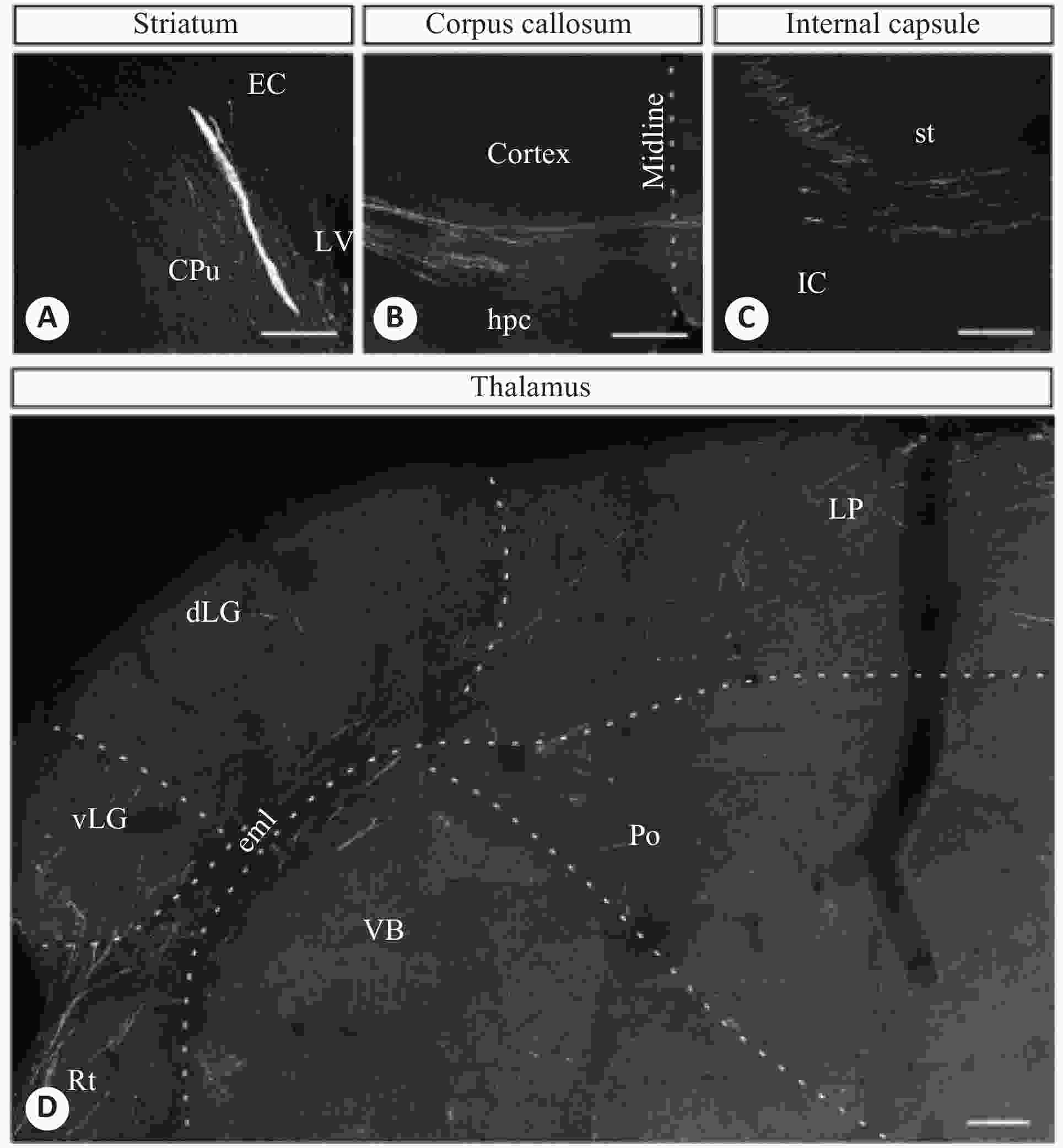
图 1 人ESC移植到新生小鼠脑部2月后不同脑部区域的轴突双光子图像
A:纹状体;B:胼胝体;C:内囊;D:丘脑[29]
Figure 1. Axonal two-photon images of different brain regions of newborn mice after human ESC transplantation
图 2 完整心脏中心肌细胞移植后的双光子图像
A:灌注rhod-2的小鼠心脏图像,包含表达EGFP的移植心肌细胞,宿主心肌细胞由于绿色的EGFP和红色的rhod-2叠加,显示为黄色;B:对A图中白线区域线扫描结果[36]
Figure 2. Two-photon images of intact central cardiac muscle cells after transplantation
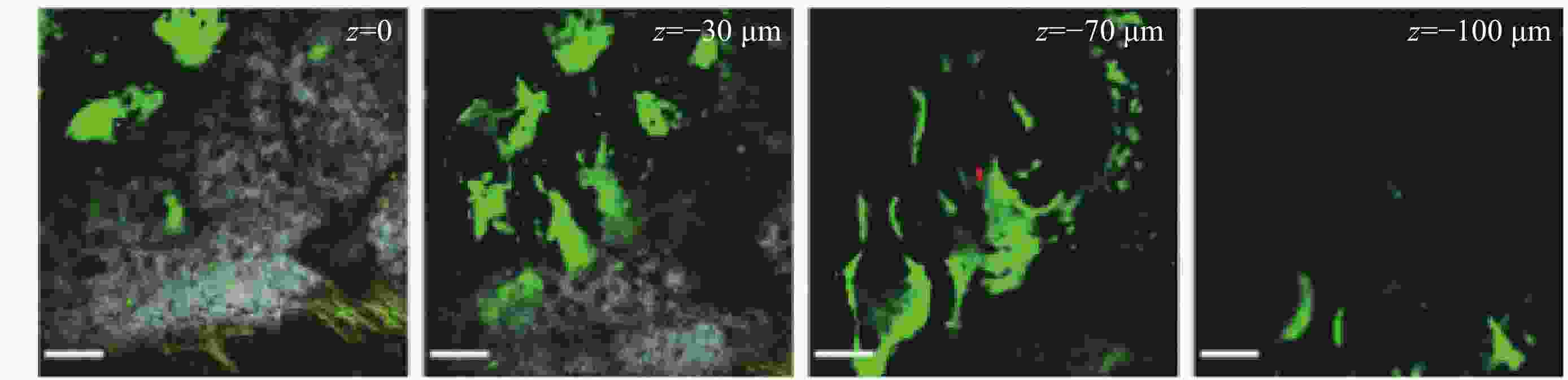
图 3 对颅骨不同深度下的二维双光子图像
白色为颅骨中胶原骨信号,红色为DiD标记的细胞,黄色为自发荧光的细胞,绿色为成骨细胞[41]
Figure 3. Two-dimensional two-photon images of the skull at different depths
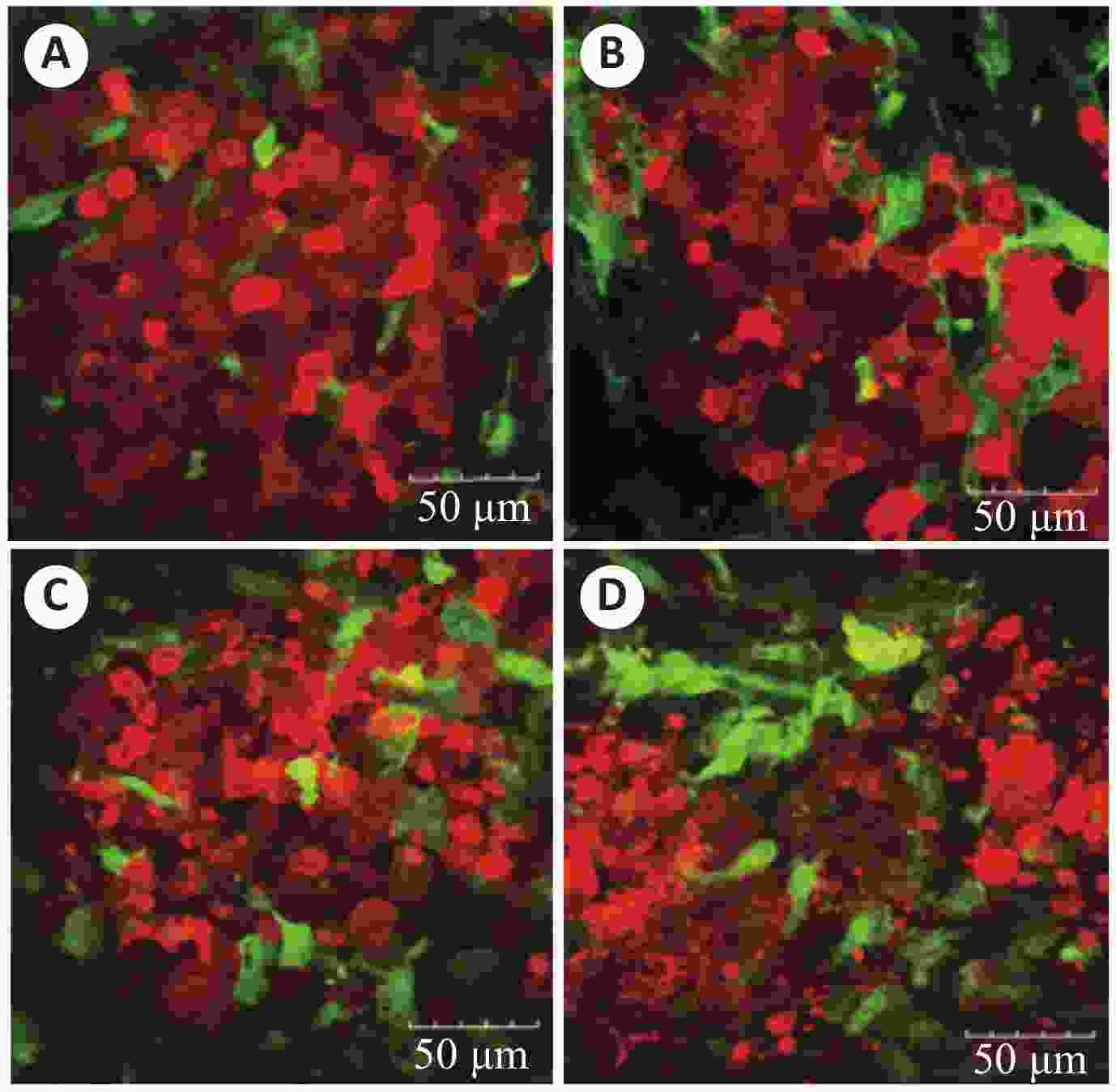
图 4 紫杉醇治疗后的肿瘤细胞双光子图像
A:对照组小鼠肿瘤细胞转移结节;B~D:治疗组小鼠肿瘤细胞转移结节,可见肿瘤细胞破碎、膨胀和细胞核聚集[44]
Figure 4. Two-photon image of tumor cells treated with paclitaxel
-
[1] Denk W, Strickler J, Webb W. Two-photon laser scanning fluorescence microscopy[J]. Science, 1990, 248(4951): 73-6. doi: 10.1126/science.2321027 [2] José A. Feijó, Moreno N Imaging plant cells by two-photon excitation[J]. Protoplasma, 2004, 223(1): 1-32. doi: 10.1007/s00709-003-0026-2 [3] Sun CK, Chu SW, Chen IS, et al. Multi-modality non-linear microscopy[C]. Lasers and Electro-Optics, 2001. Summaries of papers presented at the Conference on IEEE, 2001. [4] Ma Y, Shaik MA, Kim SH, et al. Wide-field optical mapping of neural activity and brain haemodynamics: considerations and novel approaches[J]. Philosoph Transact Royal Society Biolog Sci, 2016, 371(1705): 20150360-71. doi: 10.1098/rstb.2015.0360 [5] Grutzendler J, Gan WB. Two-photon imaging of synaptic plasticity and pathology in the living mouse brain[J]. Neurorx, 2006, 3(4): 489-96. doi: 10.1016/j.nurx.2006.07.005 [6] Sorbara CD, Wagner NE, Ladwig A, et al. Pervasive axonal transport deficits in multiple sclerosis models[J]. Neuron, 2014, 84(6): 1183-90. doi: 10.1016/j.neuron.2014.11.006 [7] Karsten K, Raphael AP, Lin L, et al. Applications of multiphoton tomographs and femtosecond laser nanoprocessing microscopes in drug delivery research[J]. Adv Drug Delivery Rev, 2011, 63(45): 388-404. [8] Oheim M, Michael DJ, Geisbauer M, et al. Principles of two-photon excitation fluorescence microscopy and other non-linear imaging approaches[J]. Adv Drug Deliv Rev, 2006, 58(9): 788-808. [9] Niesner R, Andresen V, Neumann J, et al. The power of single and multibeam two-photon microscopy for high-resolution and high- speed deep tissue and intravital imaging[J]. Biophys J, 2007, 93(7): 2519-29. doi: 10.1529/biophysj.106.102459 [10] Theer P, Hasan MT, Denk W. Two-photon imaging to a depth of 1000 microm in living brains by use of a Ti:Al2O3 regenerative amplifier[J]. Opt Lett, 2003, 28(12): 1022-4. doi: 10.1364/OL.28.001022 [11] Chung K, Deisseroth K. Clarity for mapping the nervous system[J]. Nature Methods, 2013, 10(6): 508-13. doi: 10.1038/nmeth.2481 [12] Han M, Gao X, Su J Z, et al. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules[J]. Nature Biotechnol, 2001, 19(7): 631-5. doi: 10.1038/90228 [13] Debarbieux F, Audinat E, Charpak S. Action potential propagation in dendrites of rat mitral cells in vivo[J]. J Neuroscienc Official Society Neuroscience, 2003, 23(13): 5553-64. doi: 10.1523/JNEUROSCI.23-13-05553.2003 [14] Agarwal A, Coleno M L, Wallace VP, et al. Two-Photon laser scanning microscopy of epithelial cell-modulated collagen density in engineered human lung tissue[J]. Tissue Engineer, 2001, 7(2): 191-202. doi: 10.1089/107632701300062813 [15] Lippitz M, Erker W, Decker H, et al. Two-photon excitation microscopy of tryptophan-containing proteins[J]. Proceed National Academy Sci, 2002, 99(5): 2772-7. doi: 10.1073/pnas.052662999 [16] Huang S, Heikal AA, Webb WW. Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein[J]. Biophysical J, 2002, 82(5): 2811-25. doi: 10.1016/S0006-3495(02)75621-X [17] Zipfel WR, Williams RM, Christie R, et al. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation[J]. Proceed National Academy Sci, 2003, 100(12): 7075-80. doi: 10.1073/pnas.0832308100 [18] Kwan AC, Duff K, Gouras GK, et al. Optical visualization of Alzheimer’s pathology via multiphoton-excited intrinsic fluorescence and second harmonic generation[J]. Optics Express, 2009, 17(5): 3679-89. doi: 10.1364/OE.17.003679 [19] Chalfie M, Tu Y, Euskirchen G, et al. Green fluorescent protein as a marker for gene expression[J]. Science, 1994, 263(5148): 802-5. doi: 10.1126/science.8303295 [20] Kawakami K. Transposon tools and methods in zebrafish[J]. Developmental Dynamics, 2005, 234(2): 244-54. doi: 10.1002/dvdy.20516 [21] Szalay G, Judák, Linda, Katona G, et al. Fast 3D imaging of spine, dendritic, and neuronal assemblies in behaving animals[J]. Neuron, 2016, S089(62): 7316307036-47. [22] Ji N, Freeman J, Smith S L. Technologies for imaging neural activity in large volumes[J]. Nature Neuroscience, 2016, 19(9): 1154-64. doi: 10.1038/nn.4358 [23] Yang W, Yuste R. In vivo imaging of neural activity[J]. Nature Methods, 2017, 14(4): 349-359. doi: 10.1038/nmeth.4230 [24] Renninger SL, Orger MB. Two-photon imaging of neural population activity in zebrafish[J]. Methods, 2013, 62(3): 255-267. doi: 10.1016/j.ymeth.2013.05.016 [25] Akassoglou K, Merlini M, Rafalski V A, et al. In vivo imaging of CNS injury and disease[J]. J Neurosci, 2017, 37(45): 10808-16. doi: 10.1523/JNEUROSCI.1826-17.2017 [26] Brennand KJ, Simone A, Jou J, et al. Modelling schizophrenia using human induced pluripotent stem cells[J]. Nature, 2011, 473(7346): 221-5. doi: 10.1038/nature09915 [27] Shcheglovitov A, Shcheglovitova O, Yazawa M, et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients[J]. Nature, 2013, 503(7475): 267-71. doi: 10.1038/nature12618 [28] Wen Z, Nguyen H, Guo Z, et al. Synaptic dysregulation in a human iPS cell model of mental disorders[J]. Nature, 2014, 515(6): 414-8. [29] Espuny CI, Michelsen KA, Gall D, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo[J]. Neuron, 2013, 77(3): 440-56. doi: 10.1016/j.neuron.2012.12.011 [30] Korecka JA, Levy S, Isacson O. In vivo modeling of neuronal function, axonal impairment and connectivity in neurodegenerative and neuropsychiatric disorders using induced pluripotent stem cells[J]. Molecul Cellul Neurosci, 2015, 73(1): 3-12. [31] Michelsen KA, Acosta-Verdugo S, Benoit-Marand M, el al. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells[J]. Neuron, 2015, 85(11): 982-97. [32] Falkner S, Grade S, Dimou L, et al. Transplanted embryonic neurons integrate into adult neocortical circuits[J]. Nature, 2016, 89(13): 1601-22. [33] Haissam AS, Zouein FA, Ahmed EY, et al. The march of pluripotent stem cells in cardiovascular regenerative medicine[J]. Stem Cell Res Therapy, 2018, 9(1): 201-12. doi: 10.1186/s13287-018-0947-5 [34] Lu XL, Rubart M. Micron-scale voltage and[Ca2+]i imaging in the intact heart[J]. Front Physiol, 2014, 5(3): 309-20. [35] Rubart M, Wang E, Dunn KW, et al. Two-photon molecular excitation imaging of Ca2+ transients in langendorff-perfused mouse hearts[J]. Cell Physiol, 2003, 284(6): C1654-68. doi: 10.1152/ajpcell.00469.2002 [36] Tao W, Soonpaa MH, Field LJ, et al. Functional screening of intracardiac cell transplants using two-photon fluorescence microscopy[J]. Pediatr Cardiol, 2012, 33(6): 929-37. doi: 10.1007/s00246-012-0314-8 [37] Rubart M, Tao W, Lu XL, et al. Electrical coupling between ventricular myocytes and myofibroblasts in the infarcted mouse heart[J]. Cardiovascul Res, 2017, 57(19): 2108-19. [38] Jones JS, Small DM, Nozomi N. In vivo calcium imaging of cardiomyocytes in the beating mouse heart with multiphoton microscopy[J]. Front Physiol, 2018, 9(12): 969-80. [39] Heazlewood SY, Oteiza A, Cao H, et al. Analyzing hematopoietic stem cell homing, lodgment, and engraftment to better understand the bone marrow niche[J]. Annals New York Acad Sci, 2014, 1310(1): 119-28. doi: 10.1111/nyas.12329 [40] Barrett O, Sottocornola R, Celso CL. In vivo imaging of hematopoietic stem cells in the bone marrow niche[M]. Humana Press: Progenitor Cells, 2012. [41] Scott MK, Akinduro O, Lo Celso C. In vivo 4-dimensional tracking of hematopoietic stem and progenitor cells in adult mouse calvarial bone marrow[J]. J Visual Exper, 2014, 91(12): e51683-94. [42] Duarte D, Hawkins ED, Akinduro O, et al. Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML[J]. Cell Stem Cell, 2017, 22(1): 64-77. [43] Miller MA, Weissleder R. Imaging of anticancer drug action in single cells[J]. Nature Rev Cancer, 2017, 17(7): 399-414. doi: 10.1038/nrc.2017.41 [44] Shimura T, Tanaka K, Toiyama Y, et al. In vivo optical pathology of paclitaxel efficacy on the peritoneal metastatic xenograft model of gastric cancer using two-photon laser scanning microscopy[J]. Gastric Cancer, 2015, 18(1): 109-18. doi: 10.1007/s10120-013-0334-y [45] Tanaka, Ko J. In vivo real-time imaging of chemotherapy response on the liver metastatic tumor microenvironment using multiphoton microscopy[J]. Oncol Reports, 2012, 28(5): 1822-30. doi: 10.3892/or.2012.1983 -






 下载:
下载: