Meta-analysis of effect of autologous stem cells treatment on Crohn's disease
-
摘要:
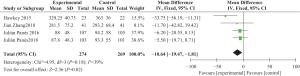
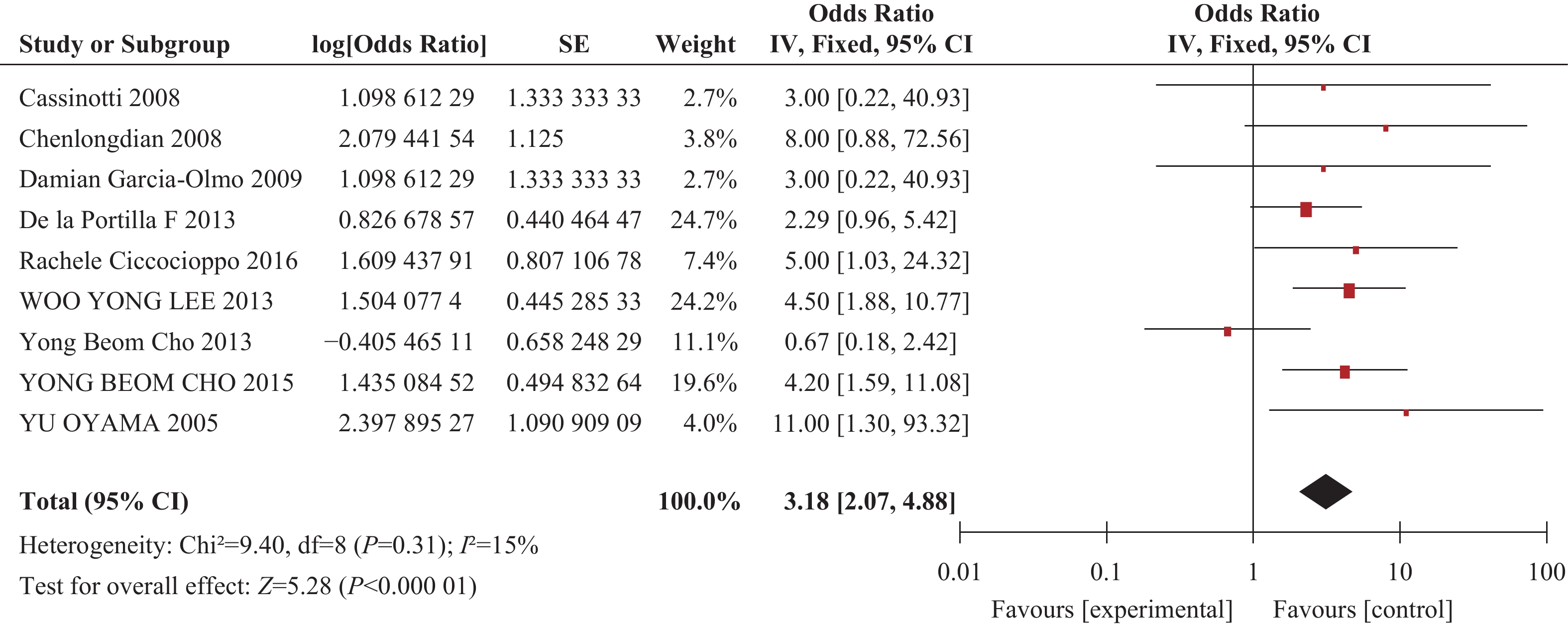
目的 评价自体干细胞治疗对克罗恩病的疗效。 方法 检索 Pubmed、MEDLINE、EBSCO、Current Content Connect、Cochrane、中国生物医学文献数据库、中国知网、万方数据库、维普数据库、谷歌学术、全国图书馆参考咨询联盟等参考文献,检索年限1963年2月~2018年7月。由2位研究者独立进行数据搜集、评价纳入研究质量、进行资料提取及交叉核对后,对纳入的研究数据使用 RevMan 5.3 软件进行多因素分析,并进行Q检验鉴定研究间异质性, 根据Q检验结果分别采用随机效应和固定效应模型合并研究,同时根据统计项目的不同进行亚组分析。 结果 检索到相关论文共1048篇,排除动物实验、病例报道及个案,最终纳入13篇论文,其中9个回顾性研究、4个病例对照研究。荟萃分析表明,通过对单臂试验统计干细胞治疗前后总体病情缓解,共纳入9篇文献,分析总体人数与有效人数危险度差,经统计分析后干细胞治疗前后危险度差值有统计学意义(RD=0.81,P<0.00001)。通过进一步统计分析纳入文献经干细胞治疗前后OR值,差异有统计学意义(OR=11,P<0.00001),但克罗恩病疾病活动指数试验组与对照组差异无统计学意义(P=0.13)。 结论 自体干细胞治疗短期内可减轻克罗恩病患者临床症状,但其安全性及其疗效确切性,有待于进一步明确。 Abstract:Objective To evaluate the effect of autologous stem cells treatment on Crohn's disease. Methods Published papers and abstracts from Pubmed, MEDLINE, EBSCO, Current Content Connect, Cochrane, China Biomedical Literature Database, China HowNet, Wanfang Database, Wipe Database, Google Academy, National Library Reference and Consulting Union database were screened from February 1963 to July 2008. After independent data collection, evaluation, data extraction and cross-check were done by 2 authors. RevMan 5.3 software was used for multivariate analysis, and Q-test for identifying the heterogeneity between two groups in the study. According to the results of Q-test, random effects and fixed effects models were used and then subgroup analysis was done in this meta-analysis. Results A total of 1048 published papers were found. After both animal experiments and case reports were excluded, 13 papers were finally included, e.g. 9 retrospective studies and 4 case-control studies. Meta-analysis showed that the RD value after stem cell therapy and before therapy was significantly different (RD=0.81, P<0.00001). The OR value after stem cell therapy and before therapy was significantly different (OR=11.00,P<0.00001), but there was no significant difference of the activity index in patients with Crohn's disease between treatment group and control group (P=0.13). Conclusion Autologous stem cells therapy might improve the clinical symptoms in patients with Crohn's disease in a short time, its safety and efficacy are still unclear. -
Key words:
- stem cells /
- Crohn's disease /
- efficacy /
- meta-analysis
-
表 1 纳入试验质量评价
研究名称 选择性 可比性 暴露分析 病例定义充分性 病例代表性 对照的选择 对照的定义 暴露的确定 是否采用相同确定方法 无应答率相同 Hawkey 2015 1 1 0 0 1 1 1 0 Julián Panés 2016 1 1 0 1 1 1 1 1 Jian Zhang2018 1 1 0 0 1 1 1 0 Julián Panés2018 1 1 0 1 1 1 1 0 -
[1] Baumgart DC, Sandborn WJ. Crohn's disease[J]. Lancet, 2012, 380(9853): 1590-605 doi: 10.1016/S0140-6736(12)60026-9 [2] Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review[J]. Gastroenterology, 2012, 142(1): 46-54 doi: 10.1053/j.gastro.2011.10.001 [3] 李冠炜, 任建安. 重视我国克罗恩病流行病学的研究[J]. 肠外与肠内营养, 2017, 24(3): 135-7 [4] Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease[J]. Gastroenterology, 2011, 140(6): 1704-12 doi: 10.1053/j.gastro.2011.02.046 [5] 陈灏珠, 钟南山, 陆再英, 等. 内科学[M]. 9版. 北京: 卫生出版社, 2018. [6] Lopez-Cubero SO, Sullivan KM, Mcdonald GB. Course of crohn's disease after allogeneic marrow transplantation[J]. Gastroenterology, 1998, 114(3): 433-40 doi: 10.1016/S0016-5085(98)70525-6 [7] Molendijk I, Bonsing BA, Roelofs H, et al. Allogeneic bone Marrow-Derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with crohn's disease[J]. Gastroenterology, 2015, 149(4): 918-27 doi: 10.1053/j.gastro.2015.06.014 [8] Becker AJ, Till JE, Mcculloch EA. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells[J]. Nature, 1963, 197(486): 452-4 [9] Lichtenstein GR, Loft J, Isaacs KL, et al. ACG clinical guideline: management of crohn's disease in adults[J]. Am J Gastroenterol, 2018, 113(4): 481-517 doi: 10.1038/ajg.2018.27 [10] Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses[J]. Eur J Epidemiol, 2010, 25(9): 603-5 doi: 10.1007/s10654-010-9491-z [11] Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for refractory crohn disease a randomized clinical trial[J]. JAMA, 2015, 314(23): 2524-34 doi: 10.1001/jama.2015.16700 [12] Panés J, García-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial[J]. Lancet, 2016, 388(151): 1281-90 [13] Panes J, Garcia-Olmo D, Van Assche G, et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with crohn's disease[J]. Gastroenterology, 2018, 154(5): 1334-8 doi: 10.1053/j.gastro.2017.12.020 [14] Zhang J, Lv S, Liu X, et al. Umbilical cord mesenchymal stem cell treatment for Crohn's disease: a randomized controlled clinical trial[J]. Gut Liver, 2018, 12(1): 73-9 doi: 10.5009/gnl17035 [15] Cassinotti A, Annaloro C, Ardizzone S, et al. Autologous haematopoietic stem cell transplantation without CD34(+) cell selection in refractory Crohn's disease[J]. Gut, 2008, 57(2): 211-7 doi: 10.1136/gut.2007.128694 [16] 陈隆典, 欧阳建, 张晓琦. 自体造血干细胞移植治疗难治性炎症性肠病10例回顾与随访[J]. 中华消化杂志, 2008, 28(7): 476-9 doi: 10.3760/j.issn:0254-1432.2008.07.014 [17] Garcia-Olmo D, Herreros D, Pascual MA, et al. Treatment of enterocutaneous fistula in Crohn's disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion[J]. Int J Colorectal Dis, 2009, 24(1): 27-30 doi: 10.1007/s00384-008-0559-0 [18] de la Portilla F, Alba F, García-Olmo D, et al. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn's disease: results from a multicenter phase I/IIa clinical trial[J]. Int J Colorectal Dis, 2013, 28(3): 313-23 doi: 10.1007/s00384-012-1581-9 [19] Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease[J]. Gut, 2011, 60(6): 788-98 doi: 10.1136/gut.2010.214841 [20] Lee WY, Park KJ, Cho YB, et al. Autologous adipose Tissue-Derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for crohn's fistula[J]. Stem Cells, 2013, 31(11): 2575-81 doi: 10.1002/stem.v31.11 [21] Cho YB, Lee WY, Park KJ, et al. Autologous adipose tissue-derived stem cells for the treatment of Crohn's fistula: a phase I clinical study[J]. Cell Transplant, 2013, 22(2): 279-85 doi: 10.3727/096368912X656045 [22] Cho YB, Park KJ, Yoon SN, et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn's fistula[J]. Stem Cells Transl Med, 2015, 4(5): 532-7 doi: 10.5966/sctm.2014-0199 [23] Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease[J]. Gastroenterology, 2005, 128(3): 552-63 doi: 10.1053/j.gastro.2004.11.051 [24] Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays[J]. Cell Stem Cell, 2008, 2(4): 313-9 doi: 10.1016/j.stem.2008.03.002 [25] Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells[J]. Science, 1999, 284(5411): 143-7 doi: 10.1126/science.284.5411.143 [26] Cho PS, Messina DJ, Hirsh EL, et al. Immunogenicity of umbilical cord tissue-derived cells[J]. Blood, 2008, 111(1): 430-8 doi: 10.1182/blood-2007-03-078774 [27] 陈月红, 杜 亮, 耿兴远, 等. 无对照二分类数据的Meta分析在RevMan软件中的实现[J]. 中国循证医学杂志, 2014, 14(7): 889-96 [28] Dietz AB, Dozois EJ, Fletcher JG, et al. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with crohn's disease[J]. Gastroenterology, 2017, 153(1): 59-64 doi: 10.1053/j.gastro.2017.04.001 [29] Ciccocioppo R, Corazza GR. Mesenchymal stem cells for fistulising Crohn's disease[J]. Lancet, 2016, 388(151): 1251-2 [30] 谢明颢, 何晓生, 朱金玲, 等. 脂肪来源间充质干细胞治疗克罗恩病的实验研究[J]. 中华胃肠外科杂志, 2015, 17(1): 58-64 doi: 10.3760/cma.j.issn.1671-0274.2015.01.015 [31] Wang M, Liang C, Hu H, et al. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis[J]. Sci Rep, 2016, 6(18): 30696-703 [32] Qiu X, Feng JR, Chen LP, et al. Efficacy and safety of autologous hematopoietic stem cell therapy for refractory Crohn's disease: A systematic review and meta-analysis[J]. Medicine (Baltimore), 2017, 96(26): e7381-7 doi: 10.1097/MD.0000000000007381 [33] Qiu Y, Li MY, Feng T, et al. Systematic review with meta-analysis: the efficacy and safety of stem cell therapy for Crohn's disease[J]. Stem Cell Res Ther, 2017, 8(1): 136-42 doi: 10.1186/s13287-017-0570-x -







 下载:
下载: